Table of Contents (click to expand)
Hot water helps to remove a metal lid from a glass jar by causing the metal lid to expand at a faster rate than the glass jar. This creates a gap between the metal lid and the glass jar, which eventually causes the lid to pop open.
The metal lid expands at a faster rate than the glass jar, which forms a gap between the metal lid and the glass jar. This gap grows as the metal lid expands, until the lid pops open from the jar.
Use a dish towel. Use a knife. Bang it on the counter. Use your hands! The Internet is full of tips on how to open a stubborn glass jar with a metal lid. However, a little science and some hot water can save you the effort of doing anything special at all. The lid just pops open. Voila!
Thermal expansion is what happens when you run a jar under hot water. But how does the process work?
Thermal Expansion
Most materials expand at high temperatures and contract at low temperatures. The phenomenon by which materials expand when exposed to heat is known as thermal expansion.
Inside The Material
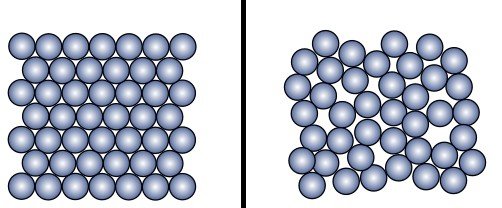
All materials are composed of basic building blocks known as atoms. Millions of atoms make up a molecule, and a material is made up of many such molecules. In solids, these molecules are arranged in a specific pattern, known as a crystal structure. They are held tightly together within the structure as a result of strong attractive forces, known as intermolecular forces of attraction. Within the crystal structure, the molecules are continuously vibrating within their fixed positions.
Effect Of Heating
Heat is the internal energy that is transferred from a body at a high temperature to a body at a low temperature. This process is also known as thermal energy transfer. When a material is heated, or receives heat from a source like hot water, the molecules in the material absorb this thermal energy.
The thermal energy does to the molecules what caffeine does to humans – it gives them an energetic boost. The molecules, which were previously just vibrating about their fixed positions, will now start bouncing up and down with this additional energy. Thus, every molecule now takes up more space than it did before to accommodate these energetic vibrations, which causes the material to expand.
Effect Of Crystal Structure
Metals have an organized crystal structure, meaning that the atoms and molecules in metals occupy specific positions. The heat therefore gets distributed more evenly over the entire material, allowing it to expand at a uniform rate. On the other hand, glass is brittle. The crystal structure in glass consists of a number of small pockets of atoms and molecules arranged very randomly. This disorganized arrangement is known as an amorphous structure and acts like an obstacle course that prevents heat energy from being distributed evenly across the material.
Also Read: When Glass Freezes, It Often Breaks. Why?
Metal Lid Vs. Glass Jar
The metal lid and the glass jar absorb different amounts of thermal energy and therefore expand at different rates. Metals, being good conductors of heat, are able to absorb thermal energy from the hot water more easily. Glass, on the other hand, being a bad conductor of heat, cannot absorb the heat energy as well. Naturally, the metal lid and the glass jar will expand at different rates as a result.
Thermal energy transfer does not necessarily involve the transfer of matter. Although hot water is flowing over the jar, neither the metal lid nor the glass jar absorb any of the water. They only absorb the heat energy that the water carries along with it.

The quantity that indicates the extent to which a material can expand upon heating is known as the coefficient of thermal expansion. Aluminum, which is normally used to make jar lids, has a thermal expansion coefficient of 23 × 10⁻6 per degree Celsius. This means that aluminum expands by 0.000023 times its original dimensions every time the temperature is raised by one degree Celsius! On the contrary, glass has a thermal expansion coefficient of only 9 × 10-6 per degree Celsius, which is much lower than aluminum.
As expected, the metal lid expands at a faster rate than the glass jar, which forms a gap between the metal lid and the glass jar. This gap grows as the metal lid expands, until the lid pops open from the jar.
Also Read: What Is Latent Heat?
Other Examples Of Thermal Expansion

- The Eiffel Tower is entirely made of wrought iron. You may not know this, but the tower gains about six inches in height every summer due to the thermal expansion of the iron within it!
- No, your house isn’t haunted. Pipes sometimes make creaking noises when you take a warm shower because the hot water causes the pipes to expand. The pipes contract once the water cools down, causing those distinct creaking noises.
- Dental fillings can often give you a toothache. When you consume something hot, the metallic fillings expand at a rate that is faster than the tooth enamel, causing tooth sensitivity and potential discomfort.
How well do you understand the article above!

