Oxygen is an important, but not necessary element for fire. There are many ways to build a fire in an oxygen-free environment.
Have you ever watched a piece of paper burn and asked yourself, “Would this be possible if there was no oxygen in Earth’s atmosphere?’ Or perhaps you’ve mused, “How do humans plan to live on Mars, as it won’t be possible to build a fire in the oxygen-depleted atmosphere of our neighbouring planet?” If you’ve asked yourself a question like this, then you’re looking at the right article, as we’ll explore the details behind this common question—Can fire occur from non-oxygenated reactions?

What Is Fire?
Sitting beside a campfire, staring into the burning pyre, you may have pondered over the nature of the fire… ‘Why is it so appealing? What are these flames made of and why do have different colours? Does fire have a chemical composition, and thus a chemical formula, like every other entity on the planet? Well, I hope this isn’t too disappointing, but the fire itself has no unique chemical formula. Fire is nothing but the outcome of a chemical reaction commonly known as combustion.

The two main components of fire are fuel and an oxidizing agent or oxidizer. Fuel is a substance that loses electrons or accepts oxygen atoms, whereas an oxidizer is a material that provides those oxygen atoms or accepts the electrons. This process of transferring electrons from the oxidizing agent to the fuel is known as oxidation, which basically constitutes combustion!
One of the most common oxidizing agents is oxygen, primarily due to its relative abundance on the planet and the two valence electrons in its outermost shell. However, oxygen is not the only oxidizing agent around.
Flames are the visible portion of the fire, and it mainly consists of gases, such as carbon dioxide, water vapour, oxygen, nitrogen and fly ash. The colour of a fire can provide a lot of information regarding what is being burned. The light produced by different elements being burned comes off as different wavelengths, and therefore appears as different colours to our eyes. An analytical chemist can easily detect a compound by the colour of its flames.
Also Read: What Determines The Color Of Flames?
Alternatives For Oxygen As An Oxidizer
As we saw earlier, oxygen plays the role of an oxidizer in the combustion reaction, but any chemical species that can replicate that role is a possible substitute for oxygen. For example, fluorine and chlorine are excellent oxidizers. Compounds containing these reactive non-metals, such as carbon trichloride, can burn metals in the absence of oxygen.
Fluoropolymers are being used to supply fluorine as an oxidizer of metallic fuels, e.g., in the magnesium/Teflon/Viton composition. Other halogens, such as bromine and iodine, can also act as oxidizing agents, but they’re less effective due to their large sizes.

Also Read: Is Oxygen Flammable?
Oxidizers In Monopropellants And Hypergolic Combinations
Monopropellants are fuels that do not require an oxidizer for combustion because the oxidizer is bound to the molecule of the fuel itself. For instance, consider a system of hydrogen and oxygen in which the hydrogen acts as the fuel and the oxygen functions as an oxidizer. Such a system would be called a bipropellant system, as the reaction would require a separate chemical species as an oxidizing agent, as opposed to a monopropellant system, which does not require any external oxygen (or any oxidizer, for that matter) for combustion. Hydrazine is the most commonly used monopropellant.
Hypergolics are combinations of two materials that ignite spontaneously without the need for an ignition source, and therefore do not require any oxygen. As they do not depend upon external ignition sources, they can be readily controlled, which makes them ideal rocket propellants.
Aerozine 50 + Nitrogen tetroxide (NTO) have been used in many American rockets, including The Titan II and Apollo Lunar Module.
Also Read: Since Fire Needs Oxygen To Burn, How Do Rockets Work In The Vacuum Of Space?
What About Nuclear Reactions?
You must be wondering by now, what about nuclear reactions? Visually, they produce the same results (heat and light) as fire and even take place on faraway stars like our sun (we always say the sun is burning) where there is no oxygen. What is happening on these stars is nothing but a nuclear fusion reaction. For those who aren’t wildly familiar with nuclear fusion, let’s dig into this idea a bit deeper.
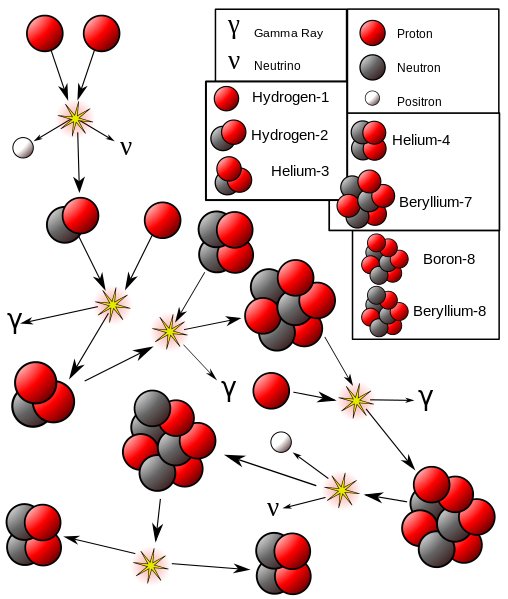
In nuclear fusion reactions, two light atomic nuclei combine to form a heavy atomic nucleus, which releases an enormous amount of light and heat energy. Fusion is what powers the sun. Atoms of deuterium and tritium (isotopes of hydrogen, Hydrogen-2, and Hydrogen-3, respectively) fuse under extreme temperature and pressure, producing a neutron and a helium isotope. The reaction is quite similar to combustion, except hydrogen serves the role of oxygen.
A Final Word
Although the feasibility of fire on any other planet remains a matter of discussion, as several factors must be considered, don’t let your imagination refrain from building settlements on Mars or planning space expeditions to explore an Earth-like planet in some other part of the universe.
Having said this, oxygen remains to be of prime importance on planet Earth for many processes, including the burning of fire, owing to its abundance and efficiency. For the past 1 million years, atmospheric oxygen levels have been declining, but fortunately, not enough to trigger any significant problems for life on Earth. However, the human race has plenty of other problems to which we are definitively contributing, so there is plenty of work to be done!
Also Read: Why Is Mars Called The “Dead Planet?”
How much do you know about fire and oxygen?

References (click to expand)
- Ericsson, N., Nordberg, Å., Sundberg, C., Ahlgren, S., & Hansson, P.-A. (2014, November). Climate impact and energy efficiency from electricity generation through anaerobic digestion or direct combustion of short rotation coppice willow. Applied Energy. Elsevier BV.
- Fission vs. Fusion – What's the Difference? - Duke Energy. Duke Energy Corporation
- Substitue for oxygen in fire. - Physics Forums. physicsforums.com
- (2010) The Handling Hazards and Lessons Learned from Use. The National Aeronautics and Space Administration
- (2010) The Handling Hazards and Lessons Learned from Use. The National Aeronautics and Space Administration
