Table of Contents (click to expand)
Maxwell’s demon is a hypothetical entity that physicist James Clerk Maxwell conjured in one of his thought experiments around 1871. The thought experiment consisted of an apparatus that would extract work from an isolated system, despite it existing in an equilibrium, at a single uniform temperature.
Basically, Maxwell’s notional entity is a sort of deus ex machina that contradicts or has cleverly devised a way around what is the most fundamental and indisputable law of the universe: the 2nd law of thermodynamics. Naturally, the notion of extracting work or energy from seemingly nothing baffled his colleagues — surely this could mean the end of tirelessly feeding coal to a ravenous steam engine? A free lunch!

Well, not really. To understand how it works, we must first understand what the law entails and why discovering a loophole beckons a riot.
Isolated Systems And The Second Law Of Thermodynamics
Thermodynamics is the branch of physics that deals with the behavior of heat and energy. Thermodynamics describes an isolated system as a region of space or a region confining an apparatus that has absolutely no contact with the external world or processes. While open or non-isolated systems are regions that confine objects that can communicate with external processes.
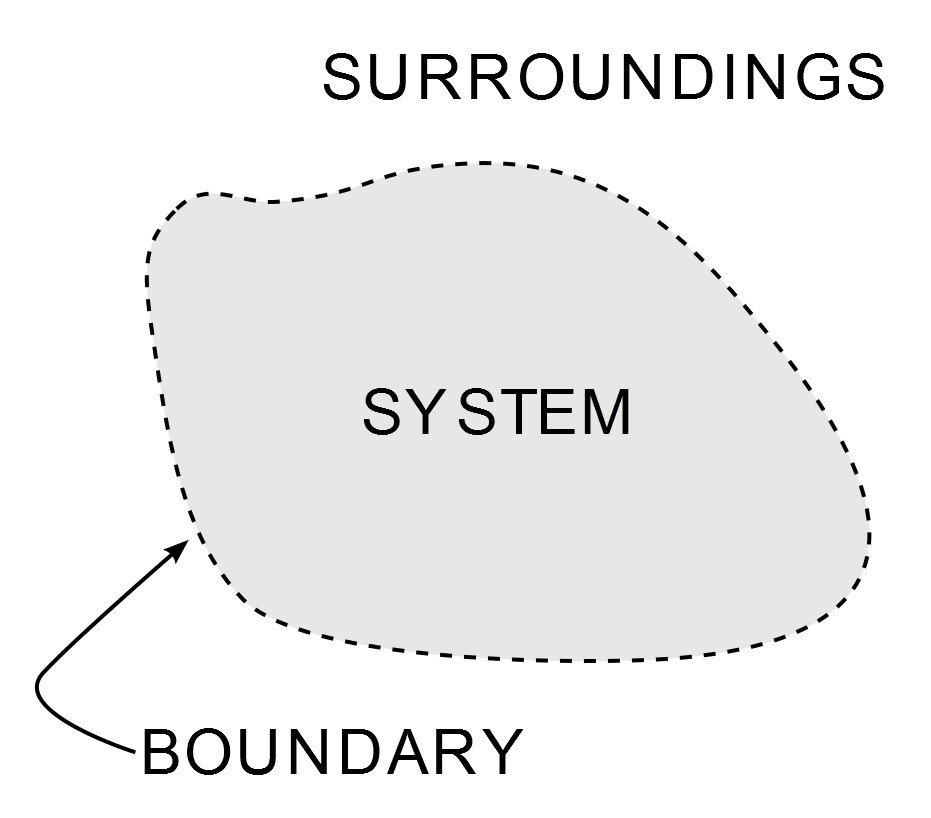
The law governs the direction of the flow of heat between two objects or regions that are incongruent in terms of their temperature. It states that two bodies of different temperatures, when acquainted with each other and isolated from their surroundings, will evolve to a thermodynamic equilibrium in which both bodies have approximately the same temperature. For that to happen, it can be logically deduced that heat must flow from the object of higher temperature to the object of lower temperature.
However, heat can flow in the opposite direction, provided it is assisted by another system (non-isolated system).
Think of this exchange like the exchange of water between two buckets. Here, the notion of temperature can be depicted by the amount of water a bucket contains. An object of higher temperature is then illustrated by a bucket with more water while an object of lower temperature by a bucket with less water.
If the buckets are now conjoined together with a narrow pipe, as shown in the figure below, you’ll observe that the water will flow from the bucket containing more water into the adjacent water until the water levels with the opening. Now no water will continue to flow, this marks the onset of equilibrium. Note that this setting represents an isolated system.
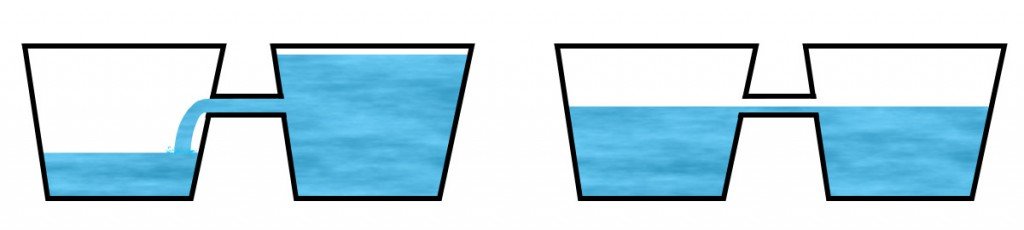
Now, water can also flow in the other direction: from the impoverished bucket to the full one, but it can only be achieved by doing work on the former: either by tilting it to a degree such that the water flows through the narrow tunnel or filling it with excess water from a third bucket, in both cases involving external help. This setting represents a non-isolated system.
This is evident in refrigerators or air conditioners where a cool breeze is obtained at the expense of warmth of another system – the condenser.
The law can also be defined in terms of entropy, a measure of statistical disorder or randomness of a system. In terms of randomness, in an isolated system, entropy will only increase. On the other hand, in a non-isolated system, witnessing a reversible process, entropy is a constant.
However, again, the constancy comes at the expense of the surrounding — the exiled heat adds entropy to the entropy as a whole of the Universe. The increase in entropy accounts for the irreversibility of natural processes.
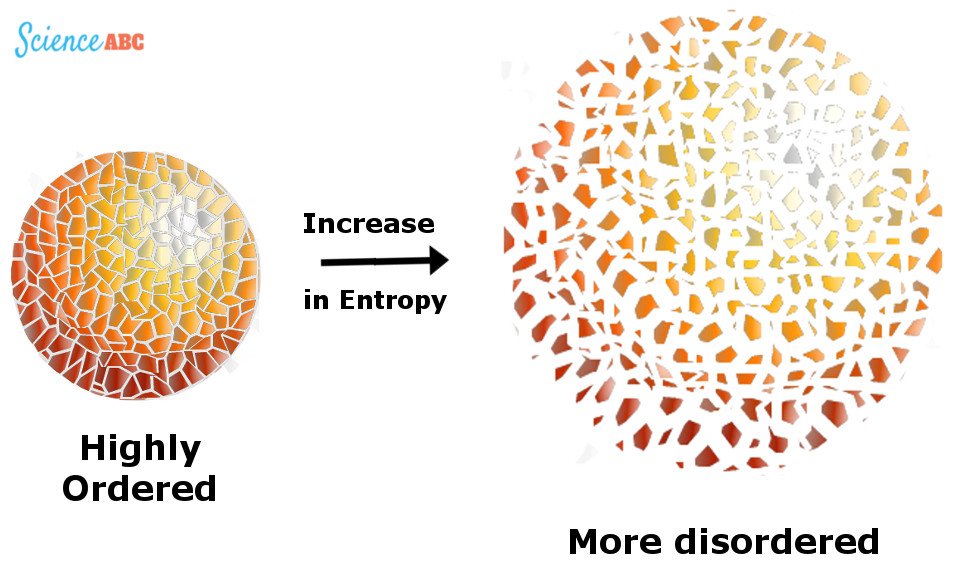
Thus, extracting energy from a system in equilibrium is impossible, but how does the devil do it?
Also Read: What Is The First Law Of Thermodynamics?
Maxwell’s Demon – The Loophole
The experiment first appeared in an exchange of letters between Maxwell and Peter Tait around 1867. It was later unveiled to the public in Maxwell’s book on thermodynamics titled Theory of Heat, published in 1872.
Although Maxwell never used the word “demon,” in his account of this experiment, an agent would open doors between chambers like a “finite being.”
However, it was William Thomson, famously known as Lord Kelvin, who first used the word “demon” to describe Maxwell’s agent, in the journal Nature in 1874. As a justification, he claimed that he intended the mediating, rather than the malevolent, connotation of the word.
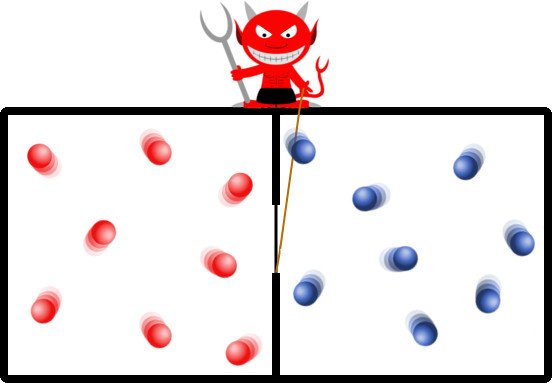
The experiment concerns an isolated system. The apparatus consists of a simple cuboid containing an arbitrary gas. The cuboid is divided into two equally sized regions with a uniform equal temperature. On the boundary of their division resides the devil, who meticulously filters the randomly squandered particles in a way that all the particles boasting higher kinetic energies end up aggregated in one region, while the remaining particles saunter around with low kinetic energies in the other region.
The demon can thus be thought of as a metaphor for a device or a machine that carefully analyzes the speed or kinetic energy of every particle inside the container. Based on its analysis, it can accurately determine which particles it must selectively usher in and which ones to play Breakout with.
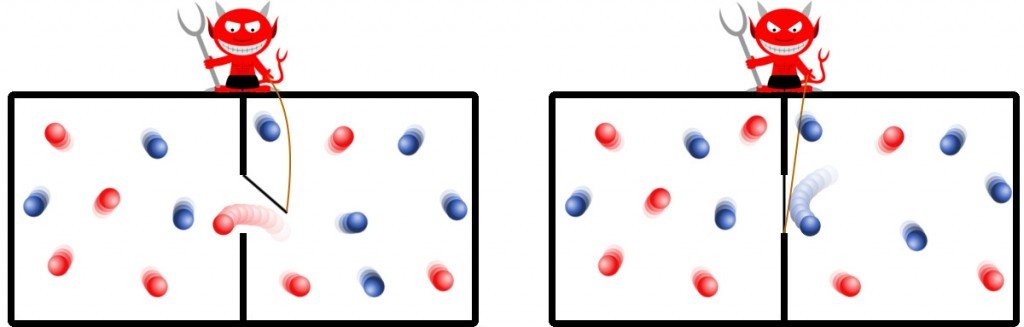
This runs contrary to the convention that the particles of a gas at a constant temperature travel around at the same speed. However, this same speed is their average speed, which means that there are particles that travel faster than that and particles that travel slower, negating each other to an average.
By this simple process, subsequently all the particles with high energies are cornered in one chamber. The demon has raised the temperature of one chamber in comparison of the other. This excess temperature or pressure can be used to power a turbine or push a piston, yes, out of perceptibly nothing. To put this another way, the demon has decreased entropy without any expenditure of work!

It is imperative to realize that the demon in his insidious ways has contradicted the law of entropy, but it still hasn’t violated the law of conservation of energy. It has merely redistributed the random kinetic energy to generate a pressure difference, such that energy can be harvested from an initially equilibrated system! The demon’s subterfuge has tricked nature itself.
Also Read: What Is Laplace’s Demon? Does This Demon Know Everything?
Can An Apparatus Like This Really Exist?
I know it’s stultifying, but it simply cannot be done. Nature isn’t one to easily fool around with. Sure, the scrupulous demon has managed to evade the oppressive policing of the 2nd law but it still cannot escape the omnipresent searchlight overhead of the 1st.
The first law asserts that no machine can do work without a heat source, and in the process, absorb some itself. Or, the efficiency of a process can never be a full 100% percent. The machines participating not only require some encouragement, but are also bound to soak some heat themselves, thereby increasing their own temperature.
The transformation of thermal energy to mechanical energy in steam engines isn’t absolute; some of the heat is absorbed by the engine itself, reducing the overall efficiency and increasing surrounding entropy.

Similarly, if the demon is a highly advanced machine that selectively seeks out certain particles, the question arises of where does it get its energy to do work from? And even if it does, the extension regarding the heat efficiency of a machine still denies the possibility of decreased entropy.
The demon or the machine would have to acquire information regarding the particles, for example, let’s say detecting photons. In the process of interacting with them, a complex machinery such as this will inevitably expend energy and soak up some heat itself, thereby increasing the net entropy back to the initial value.
The essence of the argument is that, by calculation, any demon must “generate” more entropy segregating the molecules than it could ever eradicate by the principles on which it is based. That is, it would take more thermodynamic work to detect the speed of the molecules and selectively allow them to pass through the opening between the chambers than the amount of energy gained by the temperature difference caused by the process.
However, there are certain hypotheses which attempt to explain the Maxwell demon paradox. One of them concerns Brownian motion, an idea proposed by a Polish physicist Marian Smoluchowski and further advanced by American scientist Richard Feynman through his “Brownian ratchet“. Another explanation of the paradox was proposed fairly recently by physicist Charles Bennett.
After all is said and done, one must appreciate Maxwell’s sneakiness. However, if it wasn’t for the first law, no one could save the second law from being publicly chagrined. There are no free lunches after all!
How well do you understand the article above!

References (click to expand)
- Maxwell's Demon. The Institute of Mathematical Sciences
- Maxwell's Demon. Auburn University
- Second Law of Thermodynamics - NASA. The National Aeronautics and Space Administration
- 2nd Law of Thermodynamics - Chemistry LibreTexts. LibreTexts
- Second Law of Thermodynamics - Hyperphysics. Georgia State University
