Table of Contents (click to expand)
Avogadro’s law states that under conditions of constant pressure and temperature, there is a direct relationship between the number of moles and volume of a gas. This was Avogadro’s initial hypothesis. This law was applicable to ideal gases, while real gases show a slight deviation from it.
The modern definition of Avogadro’s law is that for a particular mass of an ideal gas, the amount (number of moles) and volume of the gas are directly proportional, provided the temperature and pressure conditions are constant.
Avogadro’s Law Formula
Avogadro’s law’s mathematical formula can be written as:
V ∝ n or V/n = k
Where “V” is the volume of the gas, “n” is the amount of the gas (number of moles of the gas) and “k” is a constant for a given pressure and temperature.
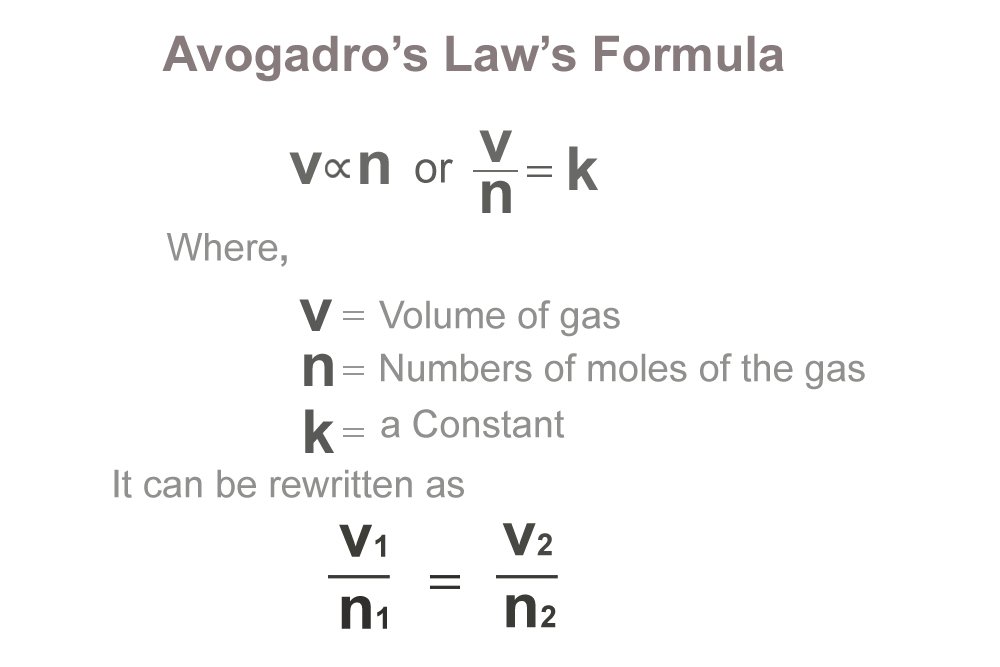
Avogadro’s law formula describes how equal volumes of all gases contain the same number of molecules, under the same conditions of pressure and temperature. In other words, it describes that equal volumes of two different gases will have the same number of molecules as long as the temperature and pressure are the same.
Amadeo Avogadro was an Italian scientist of the 19th century. He is known for making major contributions to chemistry, when it was just becoming a separate science field. His work came around the same as that of Jacques Charles (Charles Law), Robert Boyle (Boyle’s Law), etc. In fact, Avogadro’s Law, the hypothesis set by him, was among the laws on which the Ideal Gas Law is based.
An ideal gas can be defined as one in which the collisions between the molecules of the gas are elastic – i.e. there is no loss of kinetic or of momentum, and the molecules don’t have any intermolecular forces of attraction, i.e. they don’t have any interactions between them with the exceptions of the randomized collisions.
Before we get into understanding his work however, let us go over some basics.
A mole is a measure of the quantity of a substance. One mole of a substance is defined as that quantity which has as many units as the number of carbon atoms in 12 grams of C-12 carbon.
Another thing to remember is that a lot of these laws use STP or standard temperature and pressure. For STP, the value of temperature is 273.15 K (which is 0℃) while the value of pressure is 1atm or 760mmHg

Also Read: What Is Charles’s Law?
Avogadro’s Number
Avogadro’s number is the number of molecules of gas in a mole. This number is huge, the current figure being 6.022 x 1023. The unit for Avogadro’s number is mol-1. This means that the measure of the entity in question is per mole. Avogadro’s number is usually symbolised by N.
It’s interesting to note that contrary to popular belief, Avogadro’s number was not discovered by Amedeo Avogadro. The concept of mole and the determination of the value of Avogadro’s number happened after Avogadro’s death. In fact, Avogadro’s number is so called in honour of his discovery and his work.

The first person to calculate the total number of particles present in a substance was an Austrian high school teacher called Josef Loschmidt, who, after a few years, became a professor at the University of Vienna.
Using kinetic molecular theory, Loschmidt was able to estimate the number of particles present in one cubic centimeter of gas at standard conditions of pressure and temperature. The value he calculated back in 1865 is known as the Loschmidt constant today, and its value is 2.6867773 x 1025 m-3.

The term ‘Avogadro’s number’ was first used by Jean Baptiste Perrin – a French physicist. He reported an estimate of the Avogadro’s number in 1909 based on his work on Brownian motion. For the uninitiated, Brownian motion is the random, haphazard movement of microscopic particles suspended in a gas/liquid.
Accurate determination of the Avogadro’s number only became possible for the first time when Robert Millikan – an American physicist – successfully measured the charge on an electron. Prior to this, the charge on a mole of electrons was already known (it’s a constant called ‘Faraday’, which is equal to 96,485.3383 coulombs per mole of electrons).
Certain parallels have been drawn to understand how humongous this number is. One of the easiest to comprehend is if this number of unpopped kernels of popcorn were spread across the area of the United States, after popping, the popcorn would cover the country up to a depth of 9 miles (For reference, the area of the United States is 3.797 square miles!!)
You will have noticed that I mentioned the current figure of Avogadro’s number. This is because over the years since the value was first determined, different methods have been used to calculate it. While each method gives approximately the same answer, there are slight variations. Therefore, based on the most recent calculations, that is the accepted figure. The first person to make this calculation, however, was Loschmidt.
Also Read: Grams To Moles: How To Convert Grams To Moles?
Moles To Grams
Moles can be converted to grams, which is another very popular measurement of quantity, and vice versa, by the following formula
Moles = grams/molar mass
To calculate the molar mass of a substance, one has to employ the use of the ever efficient periodic table. It be calculated by simply adding the mass number of the individual atoms in the substance. For instance, if one has to calculate the molar mass of NaCl –
Mass number of Na = 22.99 g/mol
Mass number of Cl = 35.45 g/mol
Therefore molar mass of NaCl is 22.99 + 35.45 = 58.44 g/mol
Avogadro’s number has a lot of applications in chemistry and physics. Certain generalization’s have also been drawn. For instance, the volume of 1 mole of a gas at STP is 22.4L. These are very handy in calculations. Avogadro’s number is an entity used by chemists worldwide. Although he may not have determined it, his work was the precedence for these calculations.
How well do you understand the article above!

