Table of Contents (click to expand)
Cool flames are blue, spherical flames that occur in a microgravity environment. They are created by a controlled flow of oxygen and do not produce any soot.
It’s been thousands of years since our ancestors discovered fire. Even so, we can’t help ourselves but feel mesmerized by the dangerous dancing flames of a campfire. Starting and controlling fire is one of the oldest forms of chemistry practiced by humans, and over the centuries, we have formed a basic understanding of how fire behaves on terra firma. In our endless quest to push boundaries, some people decided to take fire “out of this world” to see how it behaved.
In 2012, the astronauts aboard the International Space Station started a fire. Through the Flame Extinguishing Experiment or FLEX, scientists observed something that had only been a theory until that point. Droplets of hexane were ignited in the presence of oxygen and inside the combustion chamber, blue and spherical cool flames formed. But how can a flame be cool? And why did we have to go to space to observe them for the first time? Let’s find out!

Chemistry Of Cold Flames
Flames emerge when something is on fire and the gases around it become super-heated and start to glow. The recipe for fire is quite simple, as you only need three ingredients: oxygen, fuel, and heat. This basic relationship is also known as the “fire triangle”.

As earthlings, we don’t have to worry much about the first ingredient, oxygen. At all times, our planet houses approximately 1,200,000 billion metric tons of oxygen gas. Apart from sustaining life, this oxygen-rich environment provides the perfect conditions to start a fire.
Next, we move on to fuel, which is any substance that will burn in the presence of oxygen and release energy in the process. Technically everything around us is fuel and will catch fire if it is allowed to reach high enough temperatures. However, we prefer using materials that are flammable or have low fire points as fuels, including coal, petroleum, or hexane.
The burning of fire involves a simple chemical process known as combustion. During this process, the fuel combines with oxygen to undergo several chemical reactions that emit energy in the form of light and heat. However, fuel can only react with oxygen when it’s above its ignition temperature. The excess energy required to reach this temperature and kickstart the combustion process is provided by an external source of heat. For example, the heat source for lighting a cooktop is an electric spark, whereas for a matchstick, it is the friction of the match head against the textured panel of a matchbox.
Cool flames follow the exact same chemistry, where the hydrocarbons used as fuel start burning when ignited in the presence of oxygen. Also, no, these flames do not freeze things instead of melt them. They are called “cool flames” because the temperature of these flames is quite low. An average cooking stovetop produces flames that are around 1700 ⁰C, whereas the temperature of cool flames ranges between 400 to 600⁰ C.
Also Read: Can Fire Burn Or Melt Everything?
What’s So Unique About Cool Flames?
The cool flames observed on the ISS were spherical in shape, which is almost impossible to recreate on Earth under normal conditions.
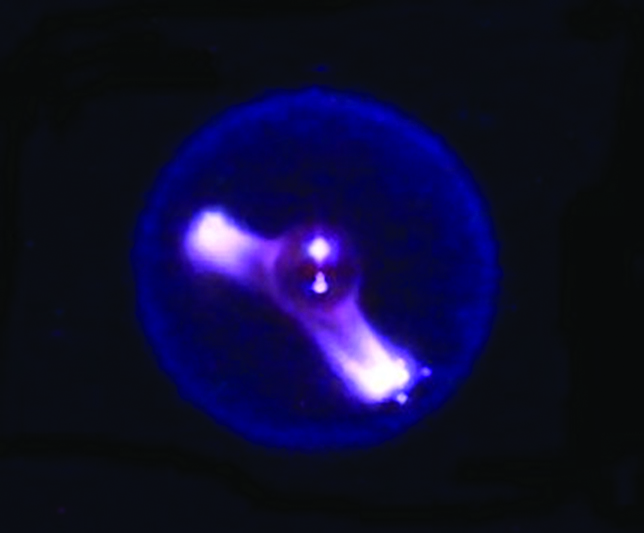
Most of us might not realize it, but gravity plays a major role in how fire behaves on our planet. When a fire is lit here, a column of air/gases around it gets heated up. By virtue of convection, the less dense hot gases rise upwards and suck in colder, fresher air to sustain the fire. This push and pull effect between the lighter hot gases and heavier cold air gives rise to the distinct teardrop shape of a flame. In space, there is no gravity to create a density gradient, which explains the spherical flames.
The spherical flames also cannot replenish their oxygen supply. An external regulator, such as a fan, is used to feed the fire. This controlled flow of oxygen gives rise to a faint blue-colored flame where fuel undergoes complete combustion to form carbon monoxide and formaldehyde, without any residual soot. A flame’s properties are slightly different in the case of self-sustained earthbound fires.
If we observe a candle flame carefully, we can spot two types of flames: the outer blue flame and the inner yellow flame. The reason for this is a difference in oxygen content and temperature. The outer blue region of a flame has the highest concentration of oxygen due to incoming fresh air from its surroundings. This makes it the hottest region of flame where the fuel (most of them carbon-based) burns completely, thus producing only carbon dioxide and water as byproducts.
The yellow region, on the other hand, has a lower temperature and lower levels of oxygen. This leads to the incomplete combustion of fuels and the formation of unburnt carbon particles—called “soot”—along with carbon dioxide and water. These soot particles then get energized by the fire and impart the typical yellow color to the flames.
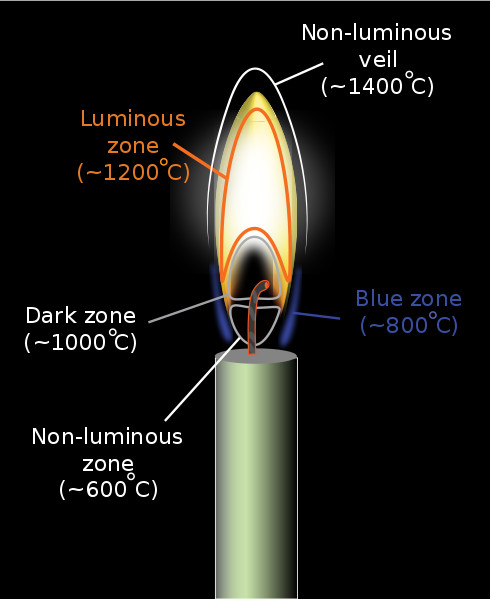
Though not very common, completely blue flames can be created on earth. All you have to do is direct enough oxygen to the fire. Equipment like Bunsen Burners and Welding torches produce almost entirely blue flames by carefully regulating their oxygen and fuel flow.
Also Read: What Determines The Color Of Flames?
What Makes The Space Flames Cool?
Firstly, because they were lit in space. Secondly, due to a slower diffusion combustion process.
In microgravity, the oxygen reaches the flame via diffusion, rather than suction, like on Earth. This slow flow of oxygen considerably reduces the temperature of the flame, which is highly dependent on the amount of fuel and oxygen available to the fire. These flames do raise the surrounding temperature or present a bright flame, due to the lack of heat radiating light-emitting ionized chemical species that are commonly found in hot flames.
Having a slow and low-temperature flame might seem like a good sign for spacecraft safety, but it’s quite the opposite. Fire on Earth is a rapid process that requires a constant and rapid flow of oxygen to continue burning. This makes it easier to start and easier to stop. Cut off the oxygen supply for a while and the fire goes out. However, that isn’t the case with cool flames. In the presence of fuel, these flames can sustain themselves for a very long time, even with a limited flow of oxygen.
Also Read: Can Spaceships Actually Explode Like They Do In Movies?
Conclusion
Very little is known about the nature of fire at lower temperatures and on places other than Earth. Unraveling the mysterious chemistry of cool flames will not only make space travel safer, but could also help us develop highly efficient soot-free internal combustion engines!
How well do you understand the article above!

References (click to expand)
- Cool Flames - NASA. The National Aeronautics and Space Administration
- An Old Theory Goes up in Cool Flames - NASA. The National Aeronautics and Space Administration
- Unlocking the Mysteries of Fire ... in Space. The A. James Clark School of Engineering
- Studying flames in microgravity is helping make combustion .... Phys.org
