Did you hate chemistry in school? If yes, then you simply weren’t taught well enough. Chemistry is fun and can explain so many different phenomena happening all around you. Different chemical reactions form different products. While some of the chemical reactions can be witnessed in daily life, such as the formation of curd and mixing sugar and coffee, others require controlled environments and catalysts. Many of these are spectacular to watch, as they produce incredible visual results.
Here are 10 of these eye-catching reactions for you!
1. Oscillating Magic Reaction
Also known as ‘the oscillating clock’, Briggs-Rauscher is one of the most common oscillating chemical reactions around. To kickstart this amazing reaction, three colorless chemicals—acidified potassium iodate (KIO3 + H2SO4), a solution of malonic acid and manganese sulphate monohydrate (HOOCCH2COOH + MnSO4. H2O) and dilute hydrogen peroxide (H2O2)—are mixed together.
The color of the resulting solution keeps oscillating between colorless, amber and deep blue for about 3-4 minutes and is really a feast for the eyes.

2. Pharaoh’s Serpent
If you want to recreate the magic of Egypt in a lab, this reaction is perfect for you. The hero of this process is Mercury(II) Thiocyanate or Hg(SCN)2. This white solid can be prepared in the lab by a precipitation reaction between mercury nitrate or mercury chloride with potassium thiocyanate. The chemical, when ignited, follows a sort of chain reaction releasing smoke and ash and growing into an olive column that looks like a snake, hence the name. The color of the serpent can be modified by adding appropriate chemicals or coloring agents. However, mercury is a very toxic chemical and should be handled carefully.

Also Read: Is The Fulminated Mercury Scene From Breaking Bad Scientifically Accurate?
3. Kaleidoscope Reaction
The beauty of this reaction lies in the fact that it was discovered by mistake. When Soviet chemist Boris P. Belousov was trying to develop a simple chemical model of the oxidation of organic molecules in living cells, he observed that the reaction did not proceed to completion and instead oscillated between different colors, looking just like illustrations of a kaleidoscope.
To replicate the result, you simply need to combine bromine with an organic acid (preferably malonic acid) in the presence of a metallic catalyst, such as magnesium or chromium, and what you observe is a stream of ripples changing colors. This reaction is also called a Belousov-Zhabotinsky reaction.

Also Read: Why Are Most Organic Compounds Colorless/White?
4. Elephant’s Toothpaste Reaction
Yes, you read that correctly! If you ever plan to get an elephant as a pet, we have the dental hygiene covered for you. The reaction is a simple decomposition reaction between hydrogen peroxide and potassium or sodium iodide along with some soap added to the reactants. It results in the production of a large amount of foam and bubbles that come out like toothpaste exploding from a tube. You can also add dyes to the solution to produce more colourful toothpaste. However, whether it cleans an elephant’s tusks or not will have to be verified by you.

Also Read: Why Does Hydrogen Peroxide Foam On Our Wounds?
5. Instant Snow
This one is one of the more straightforward reactions on the list. Sodium Polyacrylate is an interesting polymer. It has extreme water-absorbing and retaining capacity. Thereby, when water is added to the cross-linking polymer, it immediately hydrates and forms white fluffy clusters that don’t stick to each other and look just like snow. The powdered polymer can absorb as much as 500 times its mass in pure water within a few seconds. Add a little fluorescent dye and observe this in the dark: the visuals will be mesmerizing!

6. Ice Fire Reaction-Thermite And Ice
The chemical composition of thermite consists of metals such as aluminium, magnesium or zinc and oxidizers like bismuth(III) oxide or iron(II, III) oxide. This chemical compound, when ignited, can burn straight through metals and is used in railroad repairing. However, its reaction with supercooled ice or liquid nitrogen is not so productive. When thermite laid on ice is set on fire, the ice releases a large amount of energy in a very short timeframe, resulting in a loud explosion along with a dazzling light display.

Also Read: Can Fire Burn Or Melt Everything?
7. Candy Blast
Potassium chlorate, when reacted with candy or any other source of sugar, produces violet fire and a large amount of heat. These reactions on different scales have been used in pyrotechnics for centuries. Sugar has a lot of energy stored in it, which we commonly think of as calories. Our body slowly releases this energy by breaking the bonds present in sugar one by one. However, when all the bonds are broken at once, an exuberant amount of energy is released in the form of heat and light.

Also Read: What Determines The Color Of Flames?
8. The Black Magic Reaction
Calcium Gluconate is typically used to help with calcium deficiency in the body. However, when this stable-looking compound is set on fire along with some fuel, it produces some of the weirdest foam you’ve ever seen. The foam is greyish-black in color and contains mostly carbon. This is a relatively safe experiment, as the gases released usually consist of carbon dioxide and water vapour.

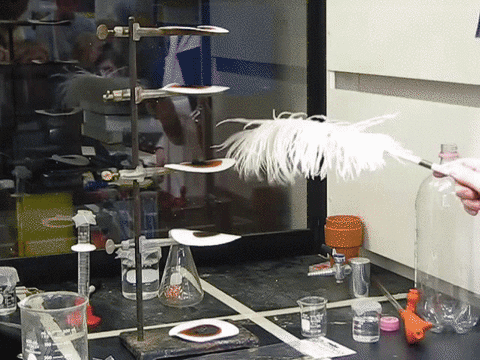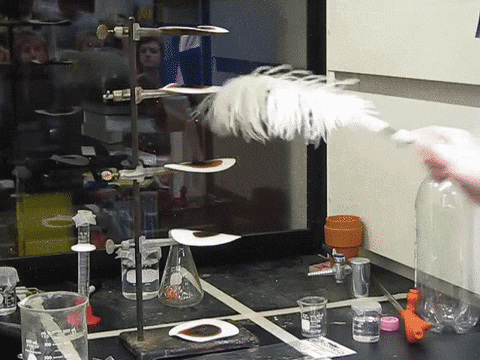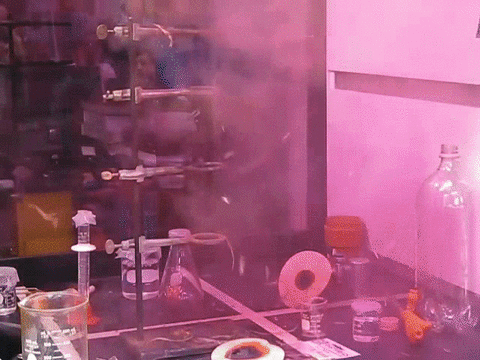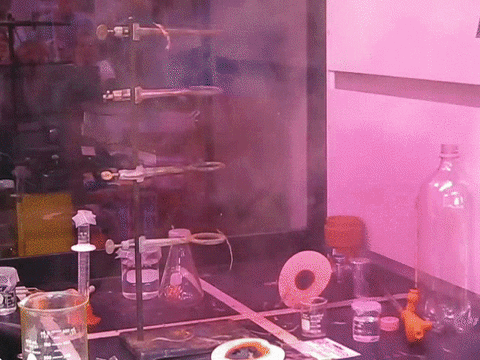
9. The Feather Explosive
Nitrogen Triiodidide is an extremely unstable compound and can be set off by the slightest movement. A feather touch or even a mosquito sitting on it can trigger its blast. Iodine is a large atom, and when three such large particles are bonded with a small nitrogen atom, there isn’t enough room for vibration. This builds up tension in the molecule that is simply waiting to be released. The blast releases violet smoke and is truly a magnificent sight to behold.
10. Invisible Water
Trust me; I wasn’t able to see this chemical when I saw this clip for the first time. Often used in magic tricks, Sulfur hexafluoride (SF6) is the densest of gases around (which means that it’s not a liquid like water, still it’s invisible!) that can carry quite a bit of weight over it. Just like most other gases, it absorbs and emits all the wavelengths of the visible spectrum, rendering it camouflaged to everything. However, unlike other gases, it is five times as heavy as air and applies much more buoyancy force on the objects placed over it.
As can be seen in the video here, the boat made of tin foil is floating in the pool of this miraculous chemical. The reaction for producing SF6 is complicated and involves reacting fluorine with sulfur in a controlled and catalytic environment. Next time you see someone performing this trick, you can explain it to your friends, or just let them be amazed!
Also Read: 5 Amazing Magic Tricks That You Can Perform Using Science

How well do you understand the article above!

