Table of Contents (click to expand)
Fulminated mercury is an unstable mercury salt of fulminic acid whose explosive properties are indirectly responsible for dynamite and directly responsible for very dynamic TV scenes!
Walter White carefully picks up the white crystal from the table. He holds it in his hand, looks at the drug dealer, and says “This is not meth”. He then slams it on the floor and kaboom! A plume of dust and fire explodes out of the room through the glass window. It leaves behind shards of flying glass and people with ringing ears.
When asked what the crystal really was, he replies “fulminated mercury, a little tweak of chemistry.”
Fulminated mercury or Mercury Fulminate is a pop culture favorite when it comes to a quickly improvised explosive that can be cooked in a lab or a basement. It has gained some screen time in shows like Law and Order and Burn Notice.
Recently in an action-adventure game, Sekiro: Shadows Die Twice, it also made an impactful appearance, but it reached stardom due to its appearance in “Breaking Bad”. This is the now-legendary show where a chemistry teacher-turned-criminal uses this substance to scare a drug dealer and get out of a dangerous situations. However, is the chemistry of that scene real or just fictional Hollywood science?
Mercury(II) Fulminate, as the name suggests is an ionic compound formed between mercury and fulminate ions. In Mercury (II) Fulminate, one unit of mercury ion (Hg2+, hence the II after mercury) combines with two units of fulminate ions (CNO–).
The -1 charge (due to an excess electron) on each of the two CNO– units compensate for Hg’s loss of two electrons (the +2 charge on Hg). As a result, they form a neutral, but very explosive compound with the chemical formula (Hg(CNO)2)
Now, let’s explore the history and try to understand the chemistry behind this eruptive compound.

Fulminates And Explosives
The chronicle of fulminated mercury dates back to the days of alchemy in the 17th century. Alchemists like Cornelius Drebbel and Johann Krunckel figured out that metals like silver or mercury, when mixed with spiritus vini (ethanol) and aqua fortis (nitric acid), gave rise to an explosive concoction.
During the later half of the 18th century, chemists mastered the art of making fulminating platinum, silver, and gold. Salts of these metals (carbonates or chlorides) were dissolved in ammonia. The precipitate obtained was then carefully dried (in the absence of heat or light). The resultant powder would explode at the slightest friction or heat.
The explosions felt almost like a thunderbolt. In fact, that is where the name fulminate was derived from, as fulmen in Latin means thunderbolt.
These powders were used as a source of entertainment and also as ammunition during war, especially fulminating gold. However, modern chemists have uncovered that what was called gold fulminate wasn’t actually a fulminate at all, as it doesn’t have the CNO– group. Instead, it is a complex salt that consists of ammonium (NH3+), nitrate (NO3–), and Chloride (Cl–) ions.
They also tried similar ammonia precipitation with mercury salts, but no one could isolate it. At least, that was true until the year 1800, when an Englishman named Edward Charles Howard became the first chemist to isolate it. He also presented a detailed method for the preparation of mercury fulminate (which by the way, he discovered accidentally).
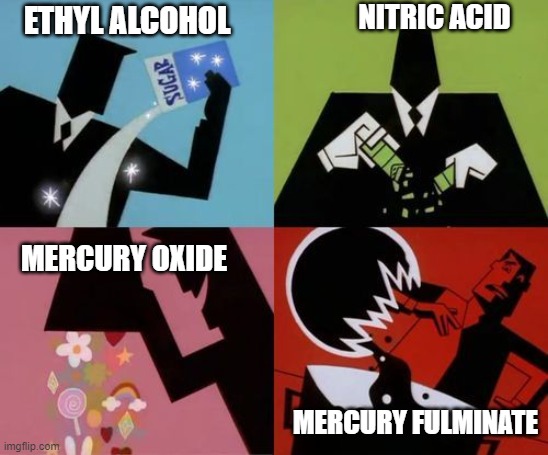
Edward was actually researching ways to synthesize muriatic acid (HCl). During his inquest, he realized that it contained hydrogen. To verify his newly acquired knowledge, he set up an experiment. To a mixture of ethyl alcohol and nitric acid, his hydrogen and oxygen sources, he added red mercury oxide as a second source of oxygen (they didn’t know that muriatic acid needed chlorine and not oxygen) and stirred them together.
All of a sudden, his reaction mixture began to bubble and release dense white smoke. After it calmed down, he could see a white precipitate settle at the bottom of his reaction container.
He crystallized the white precipitate and added a few drops of sulphuric acid to it. Initially, an effervescence was seen, followed by an explosion. He was so amazed by the explosive properties of these crystals that he tried to fulminate (detonate) them using different methods. One such experiment involved placing a few crystals (3-4) of mercury fulminate on a cold anvil and then tapping it with a hammer. A slight tap not only resulted in an explosion, but also left behind dents on both the hammer and anvil (both of which are solid metal objects).

Edward’s curiosity wasn’t quenched by the simple experiments; he wanted to try something even bigger (and probably even more dangerous). With his friend John Abernethy’s help, he loaded a gun with 11 crystals of his invention, instead of gunpowder. When the trigger was pulled, it created an explosion so intense that it ruptured the gun itself.
After many more trials of igniting it with impact, heat, or electric spark, he concluded that the firepower of mercury fulminate was due to the spontaneity of combustion.
After being convinced that the substance he created was a potent explosive, he penned down a detailed procedure on how to obtain mercury fulminate (which I won’t detail here for safety reasons). He recommended producing only 500 grains in a batch to avoid major destruction due to any accidental combustion of the product. Even after all these years, commercial manufacturers of mercury fulminate still follow a process very similar to Edward Howard’s.
Also Read: 10 Fascinating Chemical Reactions That Will Blow Your Mind
Structure Of Mercury Fulminate
The investigation into the structure of mercury fulminate began with the advent of X-ray crystallography. In the 1960s, scientists discovered that the compound was a mercury salt of fulminic acid (they share the same skeletal structure). Almost two centuries after its discovery, the bonding arrangement, and the crystal structure, of mercury fulminate, was finally determined in 2007. A group of German researchers led by Wolfgang Beck and Thomas Klapötke used the X-Ray diffraction technique to put an end to the quest.
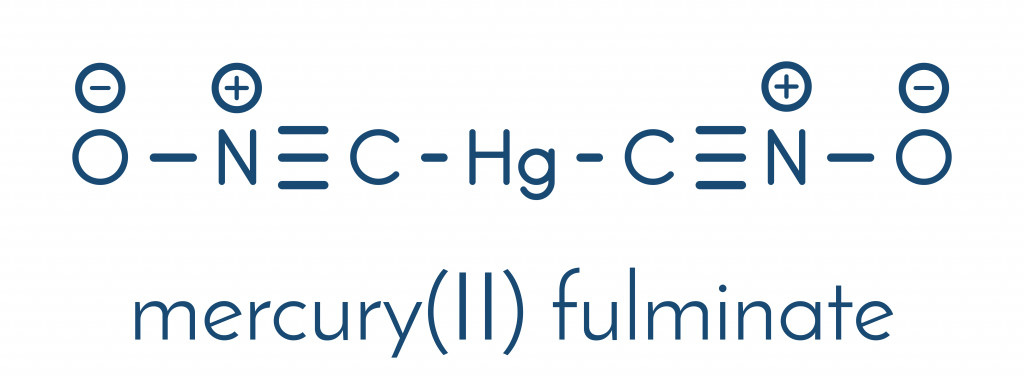

The secret behind its explosive nature lies in its structure. As you can see in the image above, both fulminic acid and mercury fulminate have the fulminate ion (CNO–), in which the carbon is attached to the nitrogen with a triple bond and the nitrogen, in turn, is attached to the oxygen atom via a single bond. This weak single bond between the N and O atoms is what makes fulminated mercury unstable and explosive.
The Fulminated Mercury Scene Of Breaking Bad
Now, coming to the first question: was the Breaking Bad scene described above scientifically accurate?
The answer is: it was partly right.
Yes, a crystal of mercury fulminate would explode when slammed to the floor. However, the crystal resembled that of a big white crystal of methamphetamine, which isn’t what mercury fulminate normally looks like. Commercial-grade crystals are usually grey to light-brown in color, due to the presence of colloidal mercury. Only an extra-pure crystal, synthesized in a professional laboratory setup, would look like the one in the show, not something that was prepared in the back of an RV.
Growing such a crystal is really difficult. Even if someone manages to grow one that size, it would be highly unstable and extremely sensitive to even the slightest of vibrations and light. The mere act of moving the crystal itself would be enough to detonate it. In the scene from Breaking Bad where Walter White takes the bag full of crystals to the drug dealer, the bag was handled quite roughly.
A crystal that size could never have survived past Walter White placing it in the bag in his lab, let alone driving with it in his car.
So, yes, they did tweak the chemistry a little bit, but the dramatic visual outcome makes this slight fib completely worth it.
Also Read: Scientific Accuracy Of The Battery-Making Scene In Breaking Bad
Conclusion
Mercury fulminate ruled the world of explosives as a primary detonator for ammunition throughout many wars. Even Alfred Nobel used it in the blasting caps used to detonate dynamite. Thus, Edward Howard’s accidental invention not only makes our crime thrillers look more theatrical, but is also indirectly the reason why Nobel Prizes exist!
How much do you know about fulminated mercury?

References (click to expand)
- Wisniak, J. (2012, April). Edward Charles Howard. Explosives, meteorites, and sugar. Educación Química. Universidad Nacional Autonoma de Mexico.
- Edward Charles Howard - Linda Hall Library. The Linda Hall Library
- (1800) On a New Fulminating Mercury. By Edward Howard ... - jstor. JSTOR
- LD TENNEY. chapter i properties of explosives - Science Madness. sciencemadness.org
- Breaking Bad IV – can a little crystal blow up a room? | The Mole. The Royal Society of Chemistry
- 300 years after discovery, structure of mercury fulminate finally .... Phys.org
- Mercury-Fulminate-Revealed - C&EN. American Chemical Society
