You do more harm than good if you drain your phone battery to 0-1% every time before recharging it. The best thing to do to extend the battery life, therefore, is to operate it in a range of 20-80%.
I am quite sure that, in today’s world, you run a better chance of finding a person who has painted himself purple dancing shirtless in the middle of the road than finding someone who doesn’t have a single electronic device in their possession… such is the ubiquity of tech gadgets these days!

Now, anyone who owns an electronic device, whether it’s a smartphone, tablet, or laptop, experiences plenty of challenges that are common to every other gadget owner. The biggest of those challenges—in my humble opinion—is the problem of battery life.
We all know of that ominous feeling when we’re just about to send that last line of a text message or replying to an ultra-important email as the phone/laptop battery powers down to its last few moments!

In a bid to tackle this omnipresent battery problem, one of the ‘tricks’ that people use is to let the battery discharge completely and then plug it in to charge it up again. The question is, does that actually help?
Does Discharging The Phone Completely Before Recharging It Improve Its Battery Life?
Short answer: No, using up all the juice in the battery before recharging does not help extend its life. Rechargeable batteries that we use in smartphones, tablets, Bluetooth headphones, laptops, and similar electronic devices are usually made of lithium-ion (Li-ion). Li-ion batteries aren’t really meant to be completely discharged before they are charged again. In fact, this practice adversely affects their longevity and is therefore counterproductive.
Since most modern electronic devices use Lithium-ion batteries, it makes sense to first understand how these batteries actually work.
Also Read: Why Does Your Smartphone Lose Charge, Even When You Don’t Use It?
How Do Lithium-ion Batteries Work?
Lithium-ion batteries, often abbreviated as Li-ion batteries, rely on Lithium ions (hence the name) to act as a crucial component in their electrochemistry. Like all batteries, a Li-ion battery also has two terminals, namely, the anode and cathode, separated by an electrolyte (a liquid that dissociates into ions in solution and thus acquires the ability to conduct electricity).
When a Li-ion battery is a part of a completed circuit (when it’s placed inside a phone/laptop), positively-charged Lithium ions move towards the negatively-charged cathode. Hence, the cathode becomes more positively charged, which causes electrons from the anode to move towards the cathode (where electrons are fewer in number).
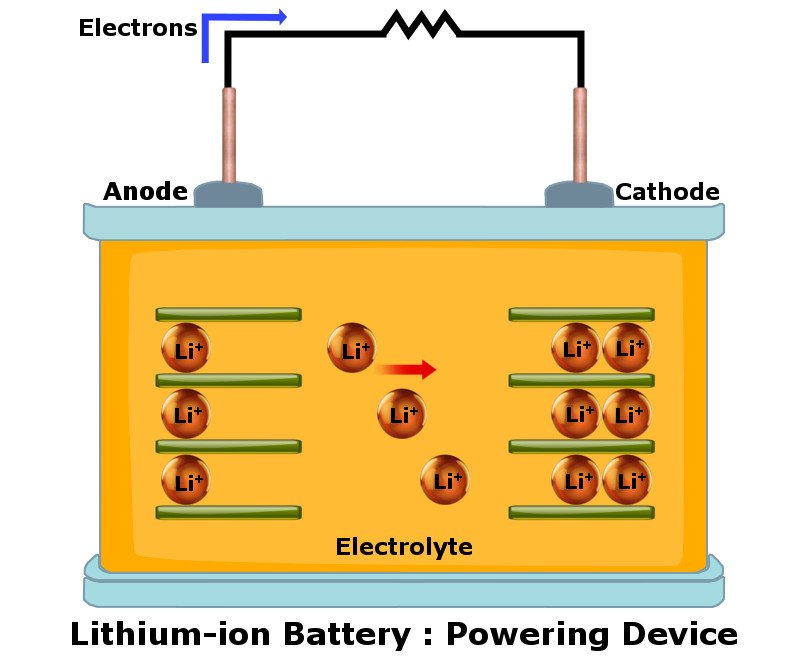
Now, the electrons want to reach the cathode as quickly as possible by taking the shortest route possible, but the electrolyte in between the electrodes prevents them from doing so. Instead, these electrons are forced to flow through the device in question (i.e., phone, laptop, etc.), which powers the device in the process.
Water Wheel Analogy
To understand how this works, think of how a water wheel rotates when fast-flowing water is made to move over it. In the case of a Li-ion battery, the fast-flowing water represents moving electrons and the water wheel represents the device (e.g., phone, laptop) that you are using.

Also Read: How Are Used Up Electric Car Batteries Recycled?
What Happens When A Li-ion Battery Is Recharged?
Since Li-ion batteries are rechargeable, they can be used over and over again by juicing them back up. This is done by connecting them to a power supply. When a Li-ion battery is connected to a charger, Lithium ions flow in the opposite direction, i.e., from the cathode to the anode, until the anode once again holds a great deal of Lithium ions. At this point, the battery shows a charge of 100%.
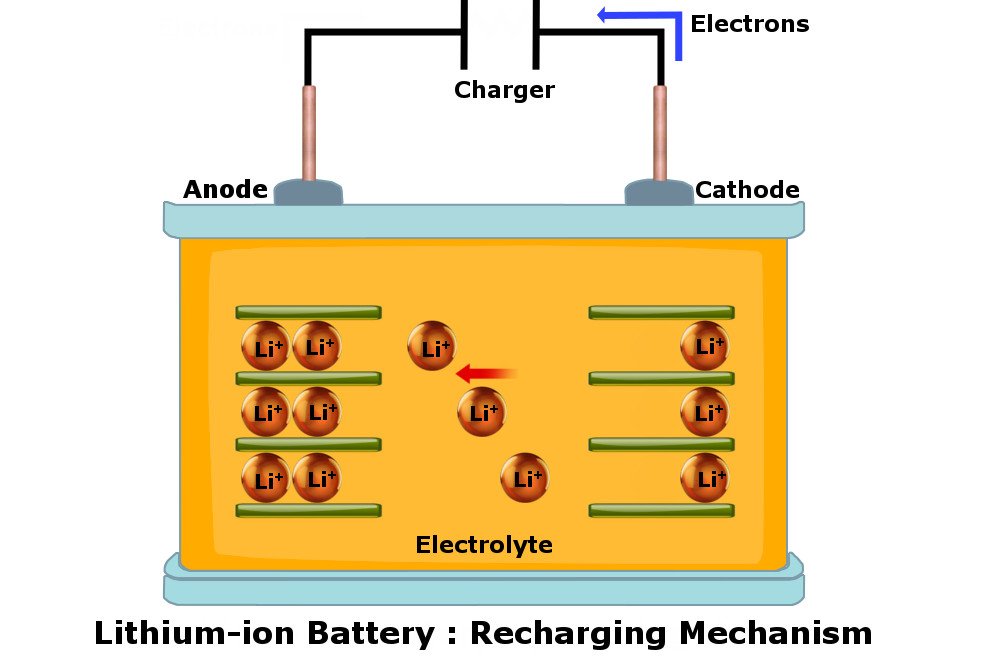
Many smartphones these days come with fast-charging technology. In fast-charging, batteries typically charge in an hour or so to 80%, and then slowly charge from 80% to 100% taking approximately another hour to reach a full charge.
Why Is Discharging A Li-ion Battery Completely Not Recommended?
Now, coming back to the question of discharging the Li-ion battery, it is not a good idea to completely drain your battery to zero before plugging it in again for a recharge. This is an unhealthy practice, as far as battery life is concerned.
No Memory Effect In Li-ion Batteries
This practice of fully draining the battery before recharge probably emerged from the usage of old nickel-based batteries, which were infamous for something called memory effect. Basically, if you fully charged a nickel-based battery, used it and let it discharge to 50%, for example, your nickel-batteries over time would develop memories that this “50%” was their actual capacity! So, after a couple of cycles of 50 to 100% charges, if you then let your nickel-based battery drain to zero and recharge, there was a good chance that the battery would charge to only 50% of its capacity due to this “memory effect”.
This isn’t the case with modern-day Li-ion batteries. They are smart enough to know their actual capacity, even if you charge and discharge randomly between any values from 0%-100%. It won’t forget its actual capacity just because you are not fully charging and fully discharging. In other words, if you just intentionally letting your Li-ion battery drain to zero just to ensure the battery does not forget its actual capacity, then you’re doing it WRONG!
Charge Cycles
Having said that, Li-ion batteries do have charge cycles. They are also known to lose their capacities marginally with each cycle. Li-ion batteries are designed to retain at least 80% of their capacity for 300-500 cycles. And the battery cycle in Li-ion battery isn’t simply one charge and discharge. Say, for example you used 50% of your fully charged battery, and recharged it again, this would constitute 0.5 cycles. Similarly, if you used up 20% of the battery, recharged it to full, and then used 80% of the battery the next day, you would have consumed 100% of a charged battery, which would mean you used one full battery cycle.
Healthy Charging Practices
Li-ions batteries hate extremities from both ends—full charge and full drain. The sweet spot to maintain battery life for a long time is to keep it somewhere in between. Carl Howe, renowned mobile analyst, gives a nice 80:20 rule to preserve battery health for an extended period: charge it to 80% and then recharge it again when it falls to around 20%.
Think of this as a healthy eating habit. It’s better to have smaller portions spread across the day, rather than just starving for hours and then having three meals all at once. Letting your battery drain to zero repetitively puts an unnecessary burden on the materials inside the battery. It could cause mechanical degradation of the cathode or side reactions with the electrode. Similarly, charging your battery to full and keeping it plugged to the charger all day can make the battery corrode faster.
As mentioned earlier, Li-ion batteries, which are mainstream these days, wear out by a tiny extent every time you move through a charge cycle. If you drain your battery from 100% all the way down to 0%, it’s likely that it could potentially degrade by up to 70% of its original capacity in just a few hundred cycles.

In a nutshell, you do your phone battery more harm than good if you let it drop to 0-1% charge every time before recharging it. The best thing to do to extend the battery life of your device is to operate it between a charge range of 20-80% whenever possible!
How much do you know about batteries of smartphones?

