Water cannot be directly used to propel vehicles. However, it can be decomposed into hydrogen and oxygen, and the isolated hydrogen can power fuel cells.
Considering our forever escalating fuel costs, filling up at a gas station can give us all sorts of wild ideas. While some friends might look to cycling as a healthy alternative, the more reasonable ones amongst us have more practical ideas: What if cars could run on water?
Can Cars Run On Water?
In its liquid state, water does not possess the mechanical or chemical energy required for propulsion. In the past, it was used with moderate success in the early years of automobiles in the form of coal-fired steam engines.
However, as the modern world faces major sustainability issues, the automobile industry is turning to other sources of fuel.
While electric cars are currently the next big thing in the industry, the debate as to whether they’re truly eco-friendly is still ongoing. Given that uncertainty, is there a way to use a resource as plentiful as water to power vehicles?
Also Read: Why Don’t We Power Cars With Solar Energy?
Hydrogen-powered Vehicles
In steam engines, water is made useful by employing coal to convert it into steam. This continues our dependency on fossil fuels, making it unsustainable for modern use, whereas hydrogen, which powers fuel cells, is a constituent element of water itself.

Hydrogen as a fuel has shown great promise in automotive propulsion. In separate designs, it has been used to generate electricity to power motors, as well as fuel combustion in modified IC engines.
It already finds use in commercial transport, such as buses and trucks, and even private transport cars. Owing to the difficulties in producing hydrogen, this format is not as popular as its electric and fossil fuel counterparts.
Also Read: Why Don’t We Just Make Water Chemically In A Lab?
The Role Of Water In Hydrogen Fuel Cells And Vehicles
To understand the role of water in hydrogen-powered vehicles, it’s crucial to understand the working of hydrogen fuel cells. Hydrogen fuel cells use chemical energy to generate electricity that powers motors. Vehicles that run on this technology are also known as Fuel Cell electric vehicles (FCEVs).
While there are many types of fuel cells, polymer electrolyte membrane fuel cells are the most commonly used.
Working Of Hydrogen Fuel Cells
Fuel cells typically consist of a cathode and an anode. Hydrogen from the fuel tank is supplied at the anode, where it reacts with a catalyst to split into its constituent subatomic particles, i.e., a proton and a neutron.
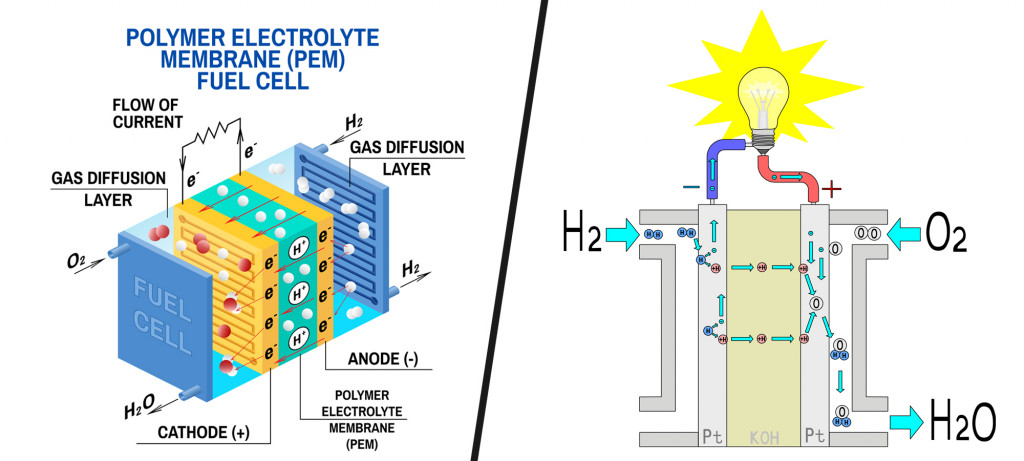
While protons migrate to the cathode, electrons are routed through an external circuit (current). This current then drives electric motors. The cathode is supplied with air, while the protons and electrons of hydrogen react with its oxygen to produce water vapor as the only end product.
To supplement the traveling range provided by hydrogen, FCEVs are equipped with brake energy recuperation technology, which stores surplus kinetic energy in an electric accumulator.
Also Read: What Are Hydrogen’s Different Colors And Why Do They Matter?
Sourcing Hydrogen – A Major Downside To Fuel Cells
Fuel cells are supplied with hydrogen stored in tanks aboard the vehicle. Like fossil fuels, hydrogen gets used up and must be replenished. Hydrogen can be either be generated in-situ, within the fuel cell, or be supplied externally. Hydrogen is expensive to produce, and transfers that infeasibility to hydrogen fuel cells.
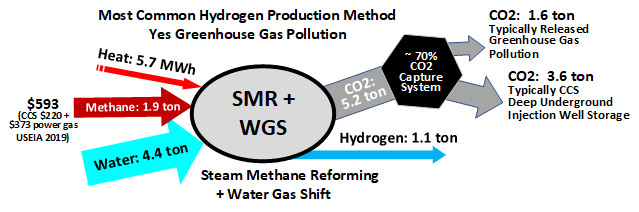
Currently, natural gas is the biggest source of hydrogen. This process involves reacting natural gas with superheated steam, resulting in the production of hydrogen, along with carbon monoxide and carbon dioxide. While the FCEV is devoid of greenhouse gas emissions, their presence in its upstream processes makes it unsuitable for long-term use.
Other sources of hydrogen include the electrolysis of water, and heat-based processes, such as the pyrolysis of organic material like feedstock. These methods are quite expensive, making them infeasible for large-scale deployment.
Importance Of Water As A Raw Material For Hydrogen Production
Water is not only the end product of a reaction in hydrogen fuel cells, but it is a source of hydrogen itself. This can create a loop, where ‘exhaust’ water from fuel cells is electrolyzed to produce the hydrogen essential for fuel cell functioning.
In such cases, dependency on hydrogen from external sources further decreases, further reducing the vehicle’s carbon footprint. While it is theoretically possible to create such a self-contained system, as per the second law of thermodynamics, it is impossible to create a self-sustaining system without incurring some loss of energy.
Thus, a loop architecture can have shortcomings that may not immediately be apparent.
Benefits Of Using FCEVs
The biggest advantage of using fuel cells is the immediate elimination of harmful tailpipe emissions. Apart from refueling times being as low as fossil fuel equivalents, they also have superior fuel economy. An average FCEV can replenish its hydrogen tank in under 5 minutes, and return fuel efficiencies in the range of 70MPGe (miles per gallon of gasoline equivalent).
This makes them a very competitive alternative to electric vehicles, which currently suffer from low range and high charging times.
Also Read: Can CNG And LPG Be Used As Car Fuels?
Challenges Of FCEVs
As promising as fuel cell vehicles are, they cannot be deployed on a large scale. The biggest challenge faced by FCEVs is the sustainable and inexpensive production of hydrogen. Its distribution and handling is another challenge that is discouraging auto makers from exploring the option too deeply.
Conclusion
While the idea of emptying a jug of water into your car’s fuel door for extended range is indeed appealing, the technology doesn’t exist just yet.
As the pressure on alternative propulsion systems mounts, to ease our reliance on fossil fuels, it is only a matter of time before we’re able to do just that!
How well do you understand the article above!

References (click to expand)
- How Do Fuel Cell Electric Vehicles Work Using Hydrogen?. The United States Department of Energy
- Hydrogen Research and Development. The United States Department of Energy
- Fuel Cell Electric Vehicles - Alternative Fuels Data Center. The United States Department of Energy
- Hydrogen Fuel Basics | Department of Energy. The United States Department of Energy
- Water Emissions from Fuel Cell Vehicles - Department of Energy. The United States Department of Energy
- A Glimpse into Hydrogen & Transportation | US EPA. The Environmental Protection Agency
- Types of Fuel Cells | Department of Energy. The United States Department of Energy
