Table of Contents (click to expand)
Fluoroantimonic acid is the world’s strongest acid, proudly standing on the pedestal slightly above carborane. However, there’s still an ongoing debate as to whether carborane is the strongest of them all. These acids are so strong that they aren’t even considered conventional acids; they are labelled superacids.
The concept of acids and bases brings to the surface of my consciousness a surge of adolescent memories. I think of lab coats, litmus papers, pungent fumes rising from oddly shaped vessels… and my poor grades.
Lab coats aren’t just recommended because that’s what the cool stereotypical forensics guy wears on every crime investigation show. The coats, along with the protective eye gear, are recommended because they protect us from harmful spills of acids, bases, salts or any other potentially harmful chemical.
Consider acids that can penetrate clothes and burn our skin. The cotton coat absorbs it, bravely sacrificing itself to protect our flesh and – more importantly – my favorite Calvin Klein shirt.

However, I have yet to witness the absurd visual of my mom wearing a lab coat and protective goggles while carefully adding vinegar to my salad. What a badass! Is she being heedless or irresponsible? Well…no. She knows, hopefully, scientifically, and not empirically, that vinegar is not a strong acid.
What makes an acid strong? In that case, what makes an acid, an “acid” at all?
What Are Strong Acids?
Acids are ionic compounds that react with a solution to produce hydrogen ions (H+). A more concise way of describing an acid is an entity that readily donates a proton. An ionized hydrogen atom, an atom without an electron, is just a proton. On the other side of the spectrum, bases are entities that readily accept this proton.
One can draw a parallel with the participants of Pass the Parcel.

If acids are the participants, then a weak acid is a participant that hesitates, or is inefficient when it comes to passing the parcel, or donating a proton. A strong acid, on the other hand, is the one that is always on its toes and is highly efficient when it comes to donating protons.
Therefore, the larger the concentration of H+ ions in a solution, the more acidic is the acid.
Measuring Acidity Objectively
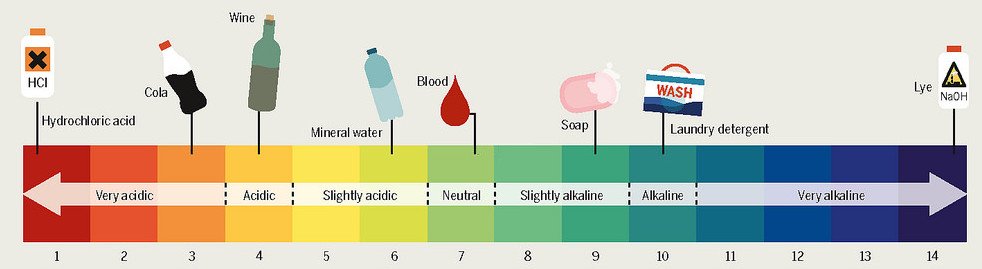
In fact, this is how acidity is measured objectively, with the help of a pH scale. The scale ranges from 1-14, where 1 depicts a very strong acid, 7 a neutral solution and 14 a very strong base.
A pH scale is a logarithmic scale. It takes the negative log (denoted by p) of the concentration of H+ ions (the H in pH). If, somehow, the concentration of hydrogen ions were to decrease, the pH would increase, as basicity would ascend.
However, it is not simply the concentration, but also the activity of these ions. The effect of these ions relies on many factors, and concentration is only one of them. That being said, for simplicity’s sake, let’s continue with the notion of concentration.
One can acquire a measure of acidity by dissolving an acid into a solution of water. The H+ cations cannot be found roaming freely; they react with water molecules (H2O) to produce hydronium ions (H3O+) and hydroxide ions (OH-).
Therefore, a higher concentration of H3O+ ions in the water signals the presence of a strong acid. The residual ion deprived of a proton or H+ ions is a base. Thus, in a sense, acids and bases naturally exist in pairs. A general representation of the reaction between water and an acid can be illustrated by this expression.
![]()
Another method of finding the relative acidity of solutions is to find the Acid Dissociation Equilibrium constant, denoted by ‘Ka’.
Referring to the above equation, we can find the magnitude of Ka with:
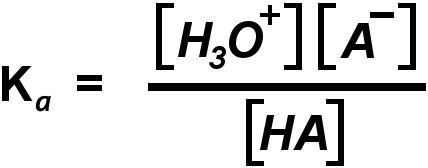
[H3O+] and [A-] represent the concentrations of hydronium ions and the separated base in moles per liter. While [HA] is the concentration of molecules that were taciturn and did not contribute any H+ ions.
For a strong acid, the product in the numerator has a larger value relative to the quantity below, in the denominator, as a strong acid would catalyze the split quickly and produce more ions, leaving fewer HA molecules behind. Consequently, the value of Ka for a strong acid will be greater than 1.
For a weak acid, on the contrary, the product in the numerator has a lower value relative to the denominator, for it is highly incompetent at producing H+ ions. Consequently, the value of Ka for a weak acid will be lower than 1.
In this way, the value of Ka determines an acid’s strength in an absolute way.
Also Read: Why Are pH Values Only In A Range Of 0-14?
What Are Some Common Strong Acids?
Hydrochloric Acid (HCl → H+ + Cl-)
Hydrochloric acid is a colorless, transparent, corrosive and very strong mineral acid. It is often referred to as “muriatic” acid and you might be familiar with it being used for cleaning bricks or concrete.
Hydrochloric acid, an ionic compound formed with hydrogen and chlorine, is a very strong acid with a pH level, depending on the concentration, wavering between -1 and 1. It almost completely dissociates in water, and its value of Ka is very large, around 1.3 × 10^6.
Hydrochloric acid has also acquired a reputation for being an excellent oxidizing agent.

Despite its strength, surprisingly, HCl constitutes the majority of our stomach (gastric) acid! It protects us from microorganisms, infections and is very important for digestion. The mucus layer protects the stomach from the acid itself. A failure in the secretion of mucus results in ulcers or heartburn.
HCl is highly corrosive and when it comes in contact, it can unapologetically burn our tissues. It can damage lungs, eyes and skin irreversibly. For that reason, it should always be handled with latex gloves and protective chemical- resistant clothing.
Nitric Acid (HNO3 → H+ + NO3-)
Nitric acid is a colorless, highly corrosive mineral acid. Nitric acid is prominently used for nitration – adding a nitrate group to a molecule, typically an organic compound. Similar to HCl, its pH value is close to -1 and dissociates almost completely in a solution of water, rendering its value of Ka large as well — 2.4 × 10.

Being an exceedingly good oxidizing agent, it reacts explosively with non-metals and organic matter. Nitration of organic compounds with nitric acid is a prominent method to produce explosives, such as trinitrotoluene (TNT). It is also used for cleaning silicon wafers or removing impurities from stainless steel.
For safety purposes, nitric acid is strictly recommended to be kept away from bases and organics, due to its pugnaciousness. Spills on skin can cause severe chemical burns and immediately decompose flesh and tissue. As a remedy, a large amount of water can be administered to the site of a burn to “neutralize” this acid.
Sulfuric Acid (H2SO4 → 2H+ + SO4^-2)
Another high school favorite, sulfuric acid is a colorless, oily, strong, corrosive mineral acid that reacts exothermically with water. It is known to singe wood or organic matter, but it is unlikely to cause a fire.

Like its cousins, sulfuric acid has a pH of around 1. Its Ka value is 1.0 × 10^3. Sulfuric acid is so dangerous that it not only causes primary burns via hydrolysis, but also secondary burns through dehydration. It can lead to blindness if it comes in contact with the eyes and irreversible throat and lung damage if swallowed.
Historically, sulfuric acid has been identified by the name “oil of vitriol”.
Also Read: Does A Strong Acid Dissolve Cold And Hot Substances At Equal Rates?
The World’s Strongest Acid
Fluoroantimonic acid is the world’s strongest acid, proudly standing on the pedestal slightly above carborane. However, there’s still an ongoing debate as to whether carborane is the strongest of them all. These acids are so strong that they aren’t even considered conventional acids; they are labelled superacids.
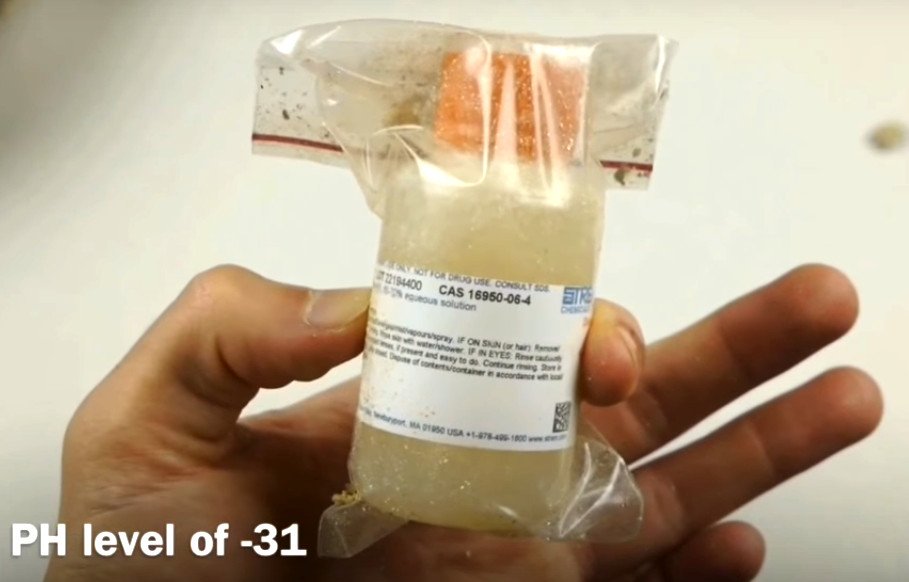
Fluoroantimonic acid has a mind-boggling pH of -31.3. It is 1 billion billion billion times more potent than gastric acid! It cannot be stored in bottles because – like Taz in Looney Tunes – it would gradually eat through the container.
The measure of its strength is such that, when in contact with a hand, it would dissolve the flesh and the bones without any hesitation. It is stored in cisterns made of Teflon. Being the strongest single bond in all of organic chemistry does have its perks – Teflon is immune to superacids.
Teflon also allows us to fry chicken more effectively, but mostly, yes, we love it because it protects us from havoc-wreaking, flesh-and-bone-dissolving superacids.
How well do you understand the article above!

