Table of Contents (click to expand)
Life has evolved to use oxygen because it is more reactive than nitrogen. Oxygen can be used in many biochemical processes that support life on Earth, while nitrogen is more difficult to break down and use.
If you paid attention in your science classes back in high school, you know that our atmosphere is made up of many gases, including nitrogen, oxygen, argon, carbon dioxide and trace amounts of several others, such as xenon, methane, krypton, hydrogen and water vapor.
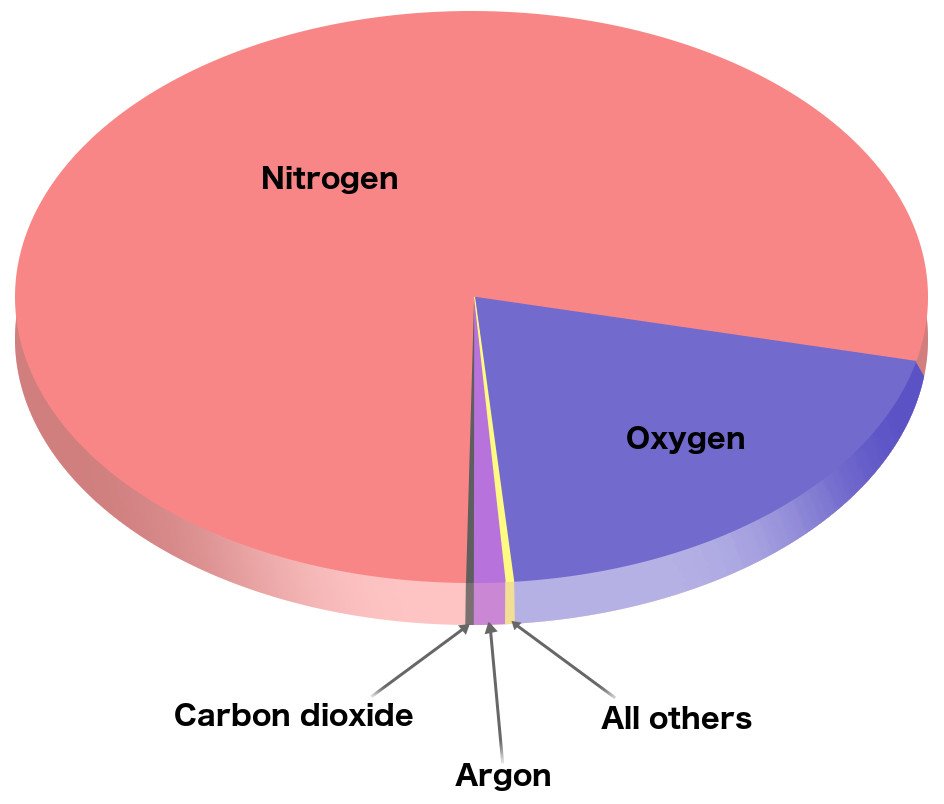
As you can see in the image above, the gas that accounts for the biggest chunk of the atmosphere is nitrogen. If we talk numbers, then 78.08% of the atmosphere is made of nitrogen. Clearly, it’s the most abundant gas in Earth’s atmosphere. The composition of atmospheres on other planets of the solar system is different from ours.
On the other hand, oxygen, one of the key players that are essential for life on Earth, stands in the second spot, accounting for almost 20.95% of the atmosphere (Source).
If you think about it, that’s a little odd, isn’t it? Life has evolved to adapt to the second most abundant gas in the environment, but not the gas that is the most abundant. What could be the reason behind that?

Before answering this question, you need to understand a thing or two about the nitrogen molecule.
Nitrogen Gas Is Quite Inert
What this means is that nitrogen doesn’t react with things very easily. More specifically, nitrogen gas exists as N2, and has a covalent triple bond.
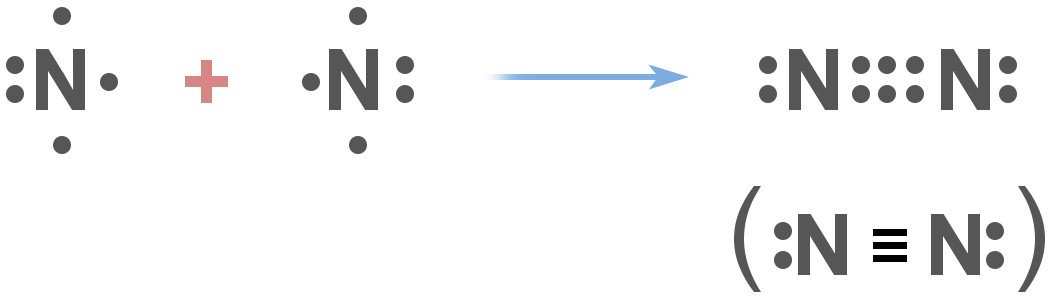
The thing about this sort of bond between two nitrogen atoms is that it’s extremely difficult to break. The bond is so strong that when chemists need to establish a ‘non-reactive atmosphere’ for their experiments, they often use nitrogen in one way or the other. The two nitrogen atoms are so closely bound to each other that it takes either a lightning bolt or certain ‘nitrogen-fixing’ bacteria in the environment that are capable of splitting them.

Oxygen on the other hand, is very reactive (in comparison to nitrogen) and can therefore be used in many biochemical processes that support life on Earth. That’s why oxygen naturally becomes the supporter of life, despite not being the most abundant gas on Earth.
Anyways, it’s not like life on Earth always depended on oxygen.
Also Read: Why Do Explosives Have Nitrogen In Them?
The Great Oxygenation Event
A couple billion years ago, Earth was not how it is now, at least in terms of the kinds of gases it had in its atmosphere back then. There were no insects, no animals, and not even leafy plants. Most of the life (primarily microorganisms like bacteria) was limited to the oceans in those days. The bacteria that thrived in that period were anaerobic, meaning they metabolized their food without using oxygen.
But then an upstart appeared, and everything changed for life on Earth. This upstart came in the form of cyanobacteria. Also known as blue-green algae, these cyanobacteria convert sunlight into energy and release oxygen as a byproduct. In more specific words, they are photosynthetic.
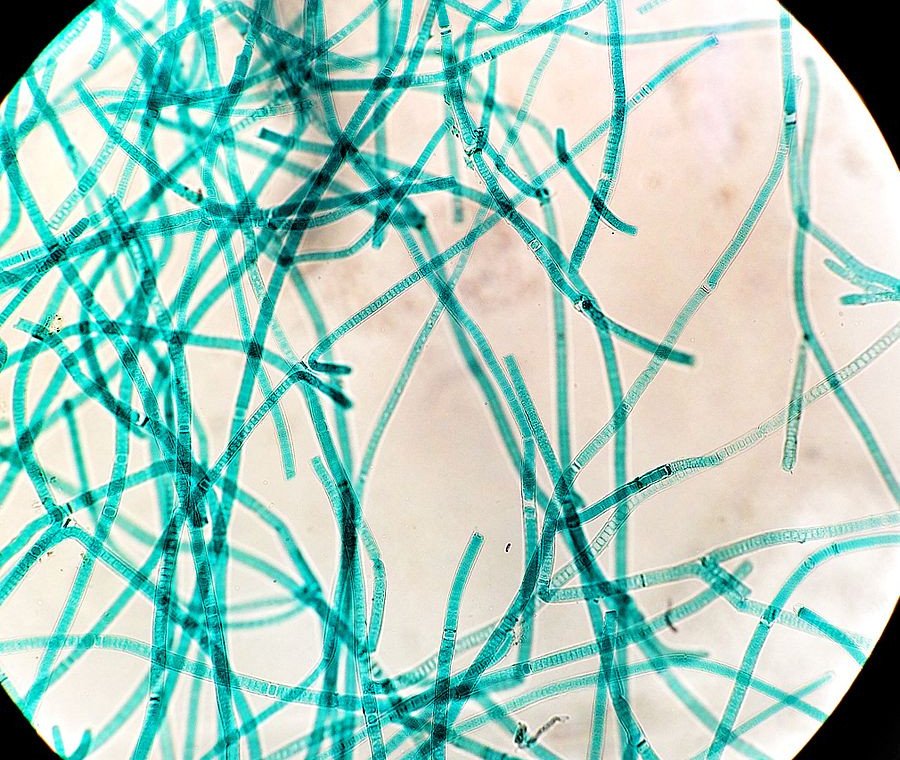
Back then, our atmosphere didn’t have as much oxygen as it does today. The little oxygen that our planet did have was either bonded with minerals or locked up within water molecules.
As cyanobacteria flourished, the supply of oxygen on the planet increased exponentially. The vast majority of anaerobic bacteria, for whom oxygen was toxic, began dying off due to this brutal onslaught of oxygen in the atmosphere. This event is what we call the Great Oxygenation Event.
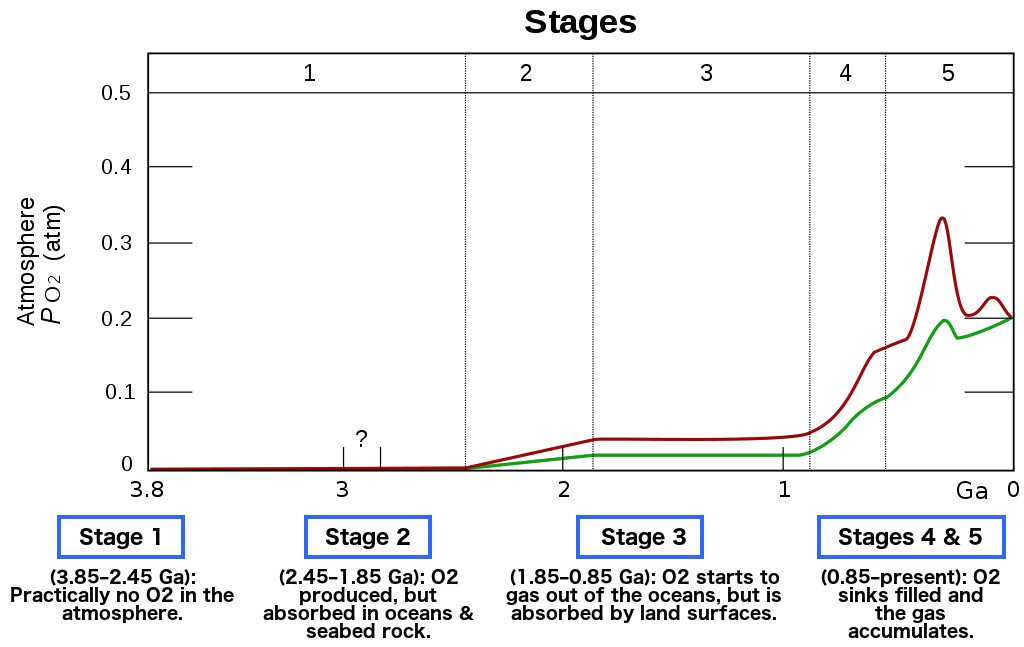
After the Great Oxygenation Event, anaerobic microorganisms died by the millions, and a buildup of oxygen soon started in the atmosphere.
It was then that other, more complex forms of life (including animals and humans) started evolving, thereby making oxygen the ‘fuel of life’.
At any rate, asking “why didn’t we evolve to use nitrogen instead of oxygen (since the former is vastly available)?” is akin to asking “why didn’t we develop cars that ran on seawater rather than gasoline, since the former is much more readily available?”
Just as seawater cannot run a car, we can’t run on nitrogen because our bodies are not ‘designed’ to do that!
Also Read: What Was The Great Oxygenation Event?
How well do you understand the article above!

References (click to expand)
- Atmospheric Composition - tornado.sfsu.edu
- What is the Atmosphere? - UCAR Center for Science Education. The University Corporation for Atmospheric Research
- The Compositon of the Atmosphere - teachertech.rice.edu
- ATMOSPHERIC COMPOSITION TEMPERATURE AND .... Central Connecticut State University
- The Oxygenation Catastrophe | The Origins Project - www.origins.asu.edu
- GeoMan's Banded Iron Page - jersey.uoregon.edu
- GeoMan's Banded Iron Page - jersey.uoregon.edu
