Table of Contents (click to expand)
Fats are used as storage molecules because they give more ATP per molecule, they take less space to store and are less heavy than glucose.
Fats are very misunderstood biomolecules. They are demonized for being unhealthy, and there was once a targeted strategy telling everyone to eat less fat.
However, fat is essential to the body.
Fat molecules are the superstars when it comes to giving the body energy, especially when your body is low on carbohydrates (like the time between meals). Then, why are fats stored as the body’s energy reserves? Why would this be different than carbohydrates, which are also a common source of energy for the body?
Fats Give More Energy When Broken Down
When it comes to comparing the amount of energy between sugars and fats, fats definitely win.
The most basic unit of all fats in the body is a fatty acid. These fatty acids are linked to other types of molecules, such as carbohydrates, phosphates, proteins or glycerol, which explains the diverse types of lipids that are found in our body. Chemically, a fatty acid is composed of a long chain of carbons (called a hydrocarbon chain) and a carboxyl group (which gives the molecule a slightly acidic nature) at one end. It looks something like this:
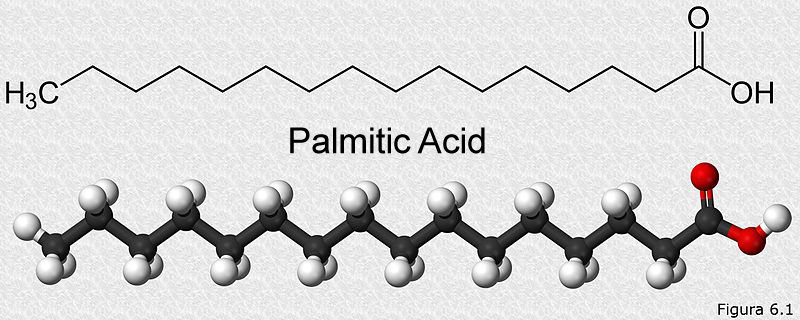
The length of the carbon chain is variable, it can be as small as 3 carbons and as long as 38 carbons. They can have only single bonds called saturated fatty acids, or they can have double bonds, which are then called unsaturated fatty acids.
Let’s consider palmitic acid, a simple 16-carbon fatty acid, pictured above. Its catabolism will occur in two steps. b-oxidation of fatty acids is the first metabolic pathway that will act on it. When our palmitic acid enters the b-oxidation pathway, it will produce a total of 28 ATP and 8 acetyl-CoA. These 8 acetyl-CoA molecules will be funneled into the citric acid cycle happening in the mitochondria. After being broken down by the citric cycle, these 8 acetyl-CoA molecules will produce 80 ATP. Therefore, the total energy given from one palmitic acid molecule is 28+80=108 ATP. In terms of calories, 1 gram of fat represents 9 kcal/g.
1 glucose molecule, on the other hand, when broken down by glycolysis and the citric cycle, yields only 40 ATP molecules. (For the uninitiated, ATP is known as the energy currency of the cell. The energy to do work comes from breaking a bond from this molecule). In terms of calories, 1 gram of carbohydrate has represents kcal/g of energy, less than half of what fat contains.
Also Read: What Are The Different Steps In Cellular Respiration?
Fats Can Be Store In Less Space Than Glucose
Besides the large energy difference in energy, fat molecules take up less space to store in the body than glucose.
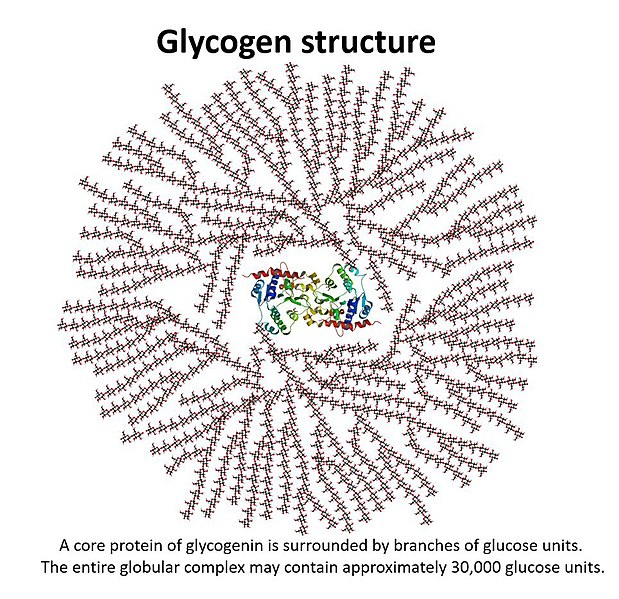
The body stores glucose by polymerizing it into a polysaccharide called glycogen. The structure of glycogen is similar to that of starch, with glycogen being more branched than starch. The glycogen is stored in the liver and muscles in b-granules. These b-granules have been recorded to be 42nm (Source: https://www.jbc.org/article/S0021-9258(20)39195-X/fulltext), but they don’t grow any more than that, as the structure would be too large to realistically fit into cells. There is approximately 100g of glucose in the liver and 400g of glucose in the muscle tissue stored as glycogen (Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2636990/).
Fats, on the other hand are stored as triglycerides (3 fatty acids linked to a glyceride molecule) in vacuoles in adipocytes. These adipocytes can grow to large sizes as more fat accumulates. As each triglyceride molecule is not covalently linked to the other in a vacuole, they can be packed close together. These adipocytes can increase (or decrease) in size to accommodate more fat needing to be stored.
Also Read: How Does Our Body Know Where To Store Fat?
Fats Need Less Water To Be Stored
Fats are hydrophobic, which literally means they are “water fearing”. This is evident in the fact that oil and water refuse to mix together. Therefore, when fat it stored, no water is associated with it. Glycogen, however, brings with it the weight of water. 1 glycogen molecule is linked to about 2 grams of water (source: Lehninger). This weight can quickly multiply as the number of b-granules increase.
The separate purposes of fat and glucose:
Glycogen, though not the preferred storage molecule of the human body, still plays an important role in maintaining blood sugar levels, especially between meals. The body maintains a stable blood sugar level so that all cells of the body get access to the energy that glucose provides. When blood glucose levels begin to deplete, glycogen is broken down to stabilize blood sugar levels back to where they started. Furthermore, some parts of the body, like the brain, only use glucose as an energy source.

Fats come into play when glycogen reserves aren’t adequate to supply the whole body with energy. Their breakdown, which is less rapid than that of glucose, will then supply cells with the energy they need. However, fats aren’t only there as energy reserves.
Lipids compose the cell membrane of every cell in the body. They are also the precursors of many hormones, such as steroid hormones. Bears and other hibernating animals have a thick layer of fat for use not only as an energy reserve during their hibernation period. Sperm whales have about 3600 kg of fat in their head alone. The oil solidifies below 37°C, the whale’s body temperature, making it denser and therefore allowing the whale to hunt in the deep sea for extended periods of time.
When it comes to the body, fats and carbohydrates have different but equally important functions. Without either of them, life would be pretty tasteless (not to mention probably non-existent!).
How well do you know fats?

References (click to expand)
- Nelson D. L.,& Cox M. M. (2017). Lehninger Principles of Biochemistry. W. H. Freeman
- Prats, C., Graham, T. E., & Shearer, J. (2018, May). The dynamic life of the glycogen granule. Journal of Biological Chemistry. Elsevier BV.
- Wasserman, D. H. (2009, January). Four grams of glucose. American Journal of Physiology-Endocrinology and Metabolism. American Physiological Society.
