Submarines are marvels of engineering that operate in the depths of the ocean, withstanding immense pressure and enabling human exploration beneath the waves.
Humans sought to explore and navigate under the sea since approximately 300 B.C. A number of methods were employed to travel underwater for research throughout history. Legend has it that the first attempt at making a submarine prototype was by Alexander the Great.
That said, venturing into the sea inside a glass barrel hardly seemed like an effective method to explore the underwater world.
In 1578 A.D., William Bourne, a British naval officer, encompassed a wooden-framed vessel covered with waterproof leather that could be rowed underwater. The first officially documented submarine, called the “Turtle” was created during the American Revolution War in 1776. It was in the late 19th century that submarines eventually evolved into practical vessels with the development of propulsion systems and more advanced technologies.
How Does A Submarine Maintain Pressure Equivalent To The Atmosphere?
Submarines are designed in a way that they maintain the same pressure inside their hulls as the atmospheric pressure at sea level.
This makes one wonder, why is it so important to maintain that matching pressure? If the internal pressure is significantly different from the external pressure, it can exert an unnatural amount of stress on the hull, leading to structural failures, leaks or even implosion.
When a submarine is on the surface, the ballast tanks are filled with air, making the vessel less dense than water and enabling it to float. However, when the submarine needs to submerge, it releases air from the ballast tanks and replaces it with water, increasing the vessel’s density. The internal pressure of a submarine is regulated by the balance between water pressure exerted on the hull and the air pressure inside.
When a submarine dives deeper, the water pressure increases, compressing the air pressure inside the hull. Thus, to equalize the pressure inside a submarine during ascent or descent, the ballast tanks are flooded or pumped out accordingly. All submarines also have internal systems called “pressure spheres” to prevent the internal air pressure from becoming too great.
Also Read: How Does A Submarine Dive, Resurface And Navigate Underwater?
What Are Pressure Hulls And Why Are They So Important?
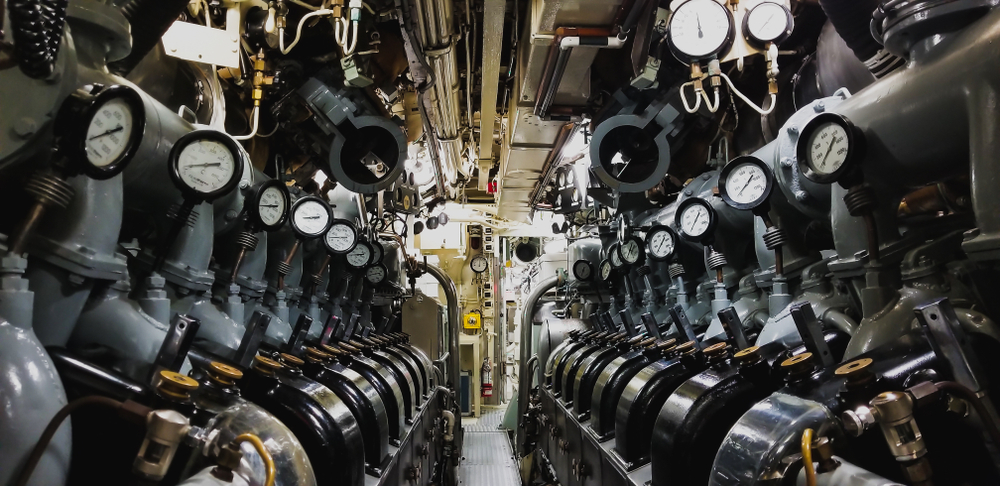
It is evident that “pressure hulls” are important structures inside a submarine, but what exactly are their functions?
The pressure hull is the main watertight structure that provides strength to the main skeleton of a submarine. It is constructed in such a way that it can withstand external pressure exerted by the ocean depths to protect the crew and systems inside.
One of the most important tasks that engineers need to be wary of when designing a submarine is making sure that the pressure hulls are resistant to leaks. It must cope with the external hydrostatic pressure without collapsing or deforming, while maintaining the overall integrity of the pressure hull.
How Do They Store Breathable Oxygen Inside A Submarine?
Submarines typically use oxygen generator canisters to produce oxygen onboard. A canister is filled with a mixture of sodium chlorate and iron powder, which when ignited, undergoes a chemical reaction and releases oxygen gas.

Since there is no direct access to the atmosphere, submarines need to store sufficient breathable oxygen for extended periods underwater. A system that generates oxygen onboard and stores it for later use is installed inside the submarines.
Underwater, one of the methods of generating oxygen is, of course, the electrolysis of water! Electrolysis splits water into hydrogen and oxygen molecules and then stores oxygen in high-pressure tanks. One can also definitely carry oxygen cylinders onboard. These cylinders store oxygen at high pressure, which can provide breathable oxygen to the people inside the submarine.
However, due to technical limitations and the energy consumed during the process, electrolysis is not a universally accepted method of oxygen generation.
Also Read: How Do People Get Oxygen And Drinking Water Inside A Submarine?
What Can Cause A Submarine To Implode?
Submarines are designed to withstand enormous external pressures; despite this, the risk of implosion still remains as one of the major concerns while constructing a submarine. Implosion occurs when the pressure outside of the submarine exceeds the structural strength of the submarine’s pressure hull, causing it to collapse inward.
When the submarine operates at great depths, the surrounding water can exert immense pressure on the pressure hull; when that pressure crosses the threshold, it becomes unbearable for the pressure hull to hold. This causes the hull to collapse inward. A number of reasons can contribute to this catastrophic failure, including weakness in the structural integrity, design flaws or even excessive depth limits.
The sudden collapse of the hull leads to nearly immediate loss of life on a submarine, the loss of the submarine and various other environmental hazards. Submarines generally undergo rigorous training and tests to ensure that such a tragic phenomenon does not occur.
A Final Word
Maintaining the same pressure as the surrounding atmosphere is crucial for the safe operation of submarines. the regulation of internal pressure is necessary to make submarines durable enough to withstand strong external water pressure to protect the crew and internal systems. The storage systems designed for storing breathable oxygen ensures a continuous supply for extended underwater missions.
Pressure hulls play an important role in providing support and strength, maintaining structural integrity, and withstanding the external pressures of deep ocean waters. Implosion, although rare, is an unavoidable concern that needs to be looked at before one decides to explore the secrets of the ocean’s deep waters.
How well do you understand the article above!

References (click to expand)
- Kim, J.-H., & Shin, H.-C. (2008, June). Application of the ALE technique for underwater explosion analysis of a submarine liquefied oxygen tank. Ocean Engineering. Elsevier BV.
- Keith, M., Haase, K. M., Schwarz-Schampera, U., Klemd, R., Petersen, S., & Bach, W. (2014, June 30). Effects of temperature, sulfur, and oxygen fugacity on the composition of sphalerite from submarine hydrothermal vents. Geology. Geological Society of America.
- KW Donald. (1979) Submarine escape breathing air. A review and analysis .... Europe PubMed Central
