Table of Contents (click to expand)
We may have many differences among us; different classes, beliefs, countries, occupations, and even tastes, but one thing that all of us have in common is our microscopic blueprint… we are all made of atoms. This isn’t just shared among fellow humans; rather, it is the case for every form of matter that exists, from bacteria and butterflies to lions, continents and even colossal stars.
Everything with a mass is an arrangement of atoms, as they are the building blocks of matter. The number of atoms in the observable universe is finite, so the atoms keep changing their arrangement to form new things. If they are finite, then we can calculate the number of total atoms in the universe, or at least reach a ballpark figure. To do that, however, we need to understand the origins of matter and the universe itself.
The Big Bang
The universe is an extremely vast place, but according to the Big Bang Theory, it expanded from an infinitesimal point. This conclusion came by backwards extrapolation of the observable expansion of the universe through time using general relativity. This extrapolation leads back to an infinitesimal point where there is infinite density and temperature, where no form of matter could possibly exist.
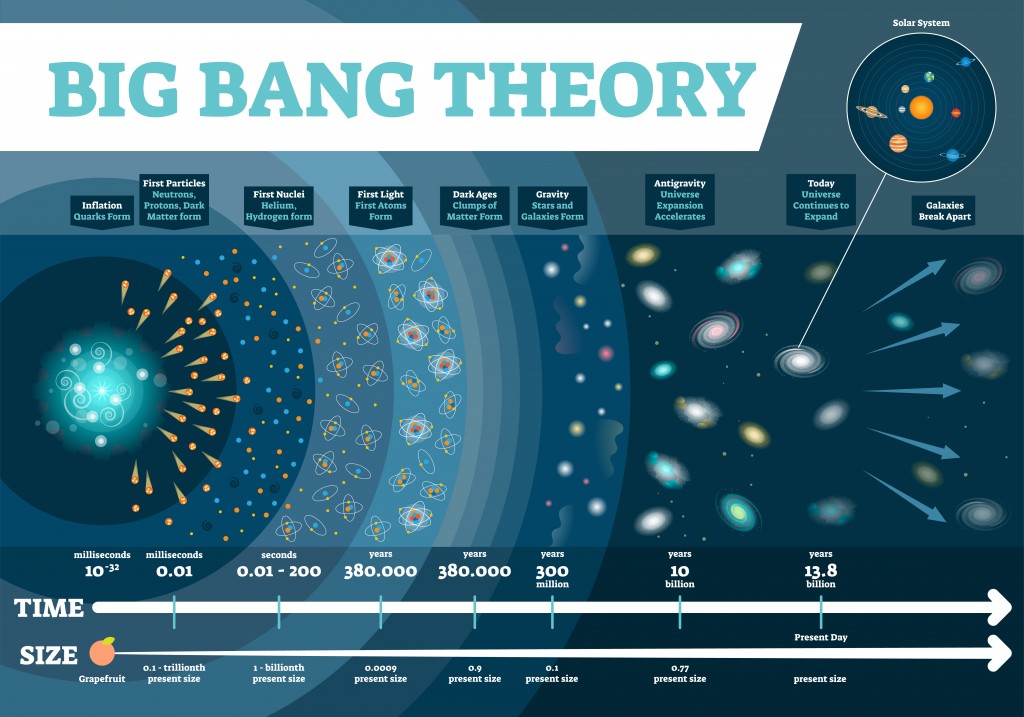
This Big Bang is traced back to a finite time – 13.8 billion years ago, the known age of our universe. The first atoms to form were hydrogen and helium, which started forming about 380,000 years after the Big Bang.
There are some physical barriers that limit our view of the universe. Namely, we can only observe the parts of the universe from areas where electromagnetic radiation has had time to reach our solar system. Thus, we don’t know what lies beyond the horizon of the observable universe, so when we calculate the number of atoms, our assumption is based on the observable universe, rather than the entire universe.
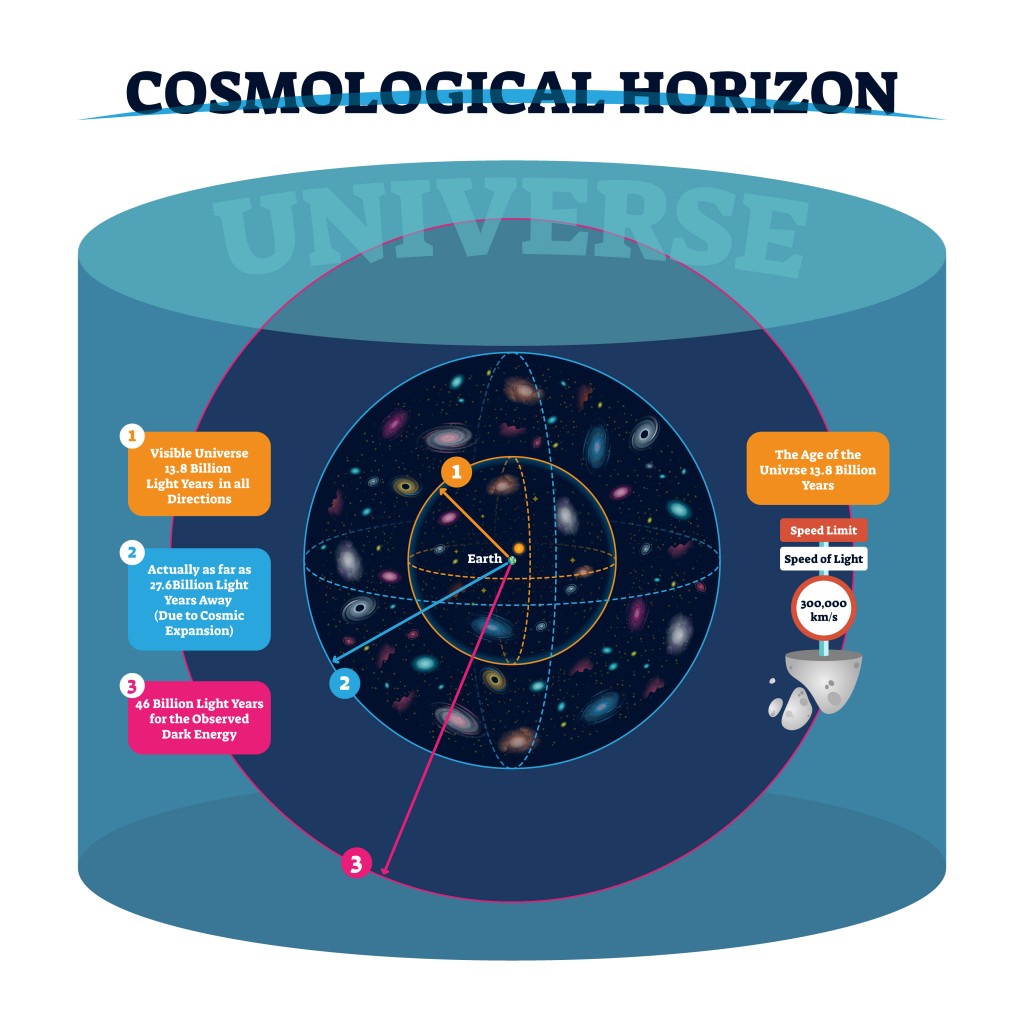
Also Read: What Existed Before The Big Bang?
The Cosmological Principle
Another thing to consider is the density and distribution of matter in the universe. Based on the cosmological principle, the universe is homogeneous and isotropic when viewed on a large scale. This means that physical laws act uniformly across the universe as a whole and should not have any observable irregularities in large-scale structures. This holds true for matter as well, which is uniformly distributed throughout the universe. This helps in estimating the number of galaxies and stars in the universe, on average. This principle also states that heavier elements were not produced by the Big Bang, but were later formed in giant stars.
Simply put, the universe looks the same for every observer from anywhere it is being looked at; the matter density is the same across large scales.
Also Read: Why Is The Universe Expanding?
The Number Of Atoms In The Observable Universe
For the purposes of this calculation, we will assume that the universe is made up of only hydrogen atoms. This will give us uniformity for the sake of easier calculation. We will start by calculating the number of hydrogen atoms in our Sun. The mass of the sun is 2.011×1033 g. The average atomic mass of the hydrogen atom is 1.008 amu. To get the number of atoms in the Sun, we would need to divide the mass of the sun by the molar mass of the hydrogen atom and multiply that by Avogadro’s number. Avogadro’s number is the number of elementary particles, such as molecules, atoms, compounds, etc. per mole of a substance; this will give us the number of atoms.
The number of atoms in the sun comes to 1.201×1057. We know that there are approximately 1011 stars in our galaxy, the Milky Way. The number of atoms in our galaxy therefore comes to 1.201×1068 when the number of stars is multiplied by the number of atoms in the sun.
For the observable universe, we know that there are approximately 1011 galaxies. We will find the number of atoms in the universe by multiplying the number of atoms in our galaxy by the number of galaxies in the universe. The total number of atoms in the universe then comes to… 1078!
Another way to do this calculation is by calculating the mass of the universe. We know on average that there are about 400 billion galaxies in the observable universe, which comes to around 1.2 x 1023 stars or over 100 sextillion. On average, a star weighs around 1035 grams, which makes the total mass of the universe 1058 or 1.2 x 1052 metric tons. Each gram of matter contains about 1024 protons, which is the same if we assume all the atoms are hydrogen, as a hydrogen atom contains only one proton. The total number of hydrogen atoms will then total 1086 or one-hundred thousand quadrillion vigintillion.
These rough calculations range from 1078 to 1086. In layman’s term, this is about ten quadrillion vigintillion and one-hundred thousand quadrillion vigintillion atoms. Better yet, it would look like 10 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 atoms… lots of zero’s indeed!

Even though that number of atoms seems like a lot, matter only comprises 4.9% of the observable universe. The rest is made up of 68.3% dark energy and 26.8% dark matter. Dark energy and dark matter are also postulated to be uniformly distributed or organized in clumps, just like normal matter, throughout the universe. Their properties are largely unknown, but it is agreed upon that they help in the expansion of the universe.
We must note that these are rough estimates based on many broad assumptions. As science progresses further, we will devise even better ways to make more accurate estimates, namely by simulating the universe in computers.
In any case, the atoms will keep transforming into different arrangements of other things, just like the atoms that were once in a star now make up you and me. With all of this in mind, it will be hard to ever forget that you’re made of star-stuff!
Also Read: Where Did The Elements Come From?
Test how well do you know about the atoms in our universe

References (click to expand)
- WHERE DID ALL THE ELEMENTS COME FROM?? - www.haystack.mit.edu
- Universe Exploration: From the Big Bang to Life. space.mit.edu
- Mass, Size, and Density of the Universe. The University of Massachusetts Amherst
- Simulating the universe as the ultimate Big Data problem. The Kavli Institute for Particle Astrophysics and Cosmology
