Table of Contents (click to expand)
Soap is effective for washing clothes because it contains surfactants, which help lower the surface tension of water molecules, thus ‘persuading’ them to wet things more uniformly. In addition, the hydrophobic tail of the soap molecule attaches itself to grime and dirt, while the hydrophilic head gets affixed to water molecules. Therefore, when the dirty clothes are put inside a washing machine or swished vigorously by hand, dirt is pulled away from the cloth and washed away in the water, leaving the cloth sparkling clean.
Soap is great for washing clothes, and everyone knows that,
But does anyone really know why? Why is soap the magical substance that can wash clothes? Why can’t anything else do the job, from tomato ketchup to a bottle’s worth of Coca-cola? What’s so special about soap that makes it able to clean our clothes to the point that (in some cases) they look as good as new?
Before we get into that, we should probably answer a more basic question…
What Is Soap?
It might sound like a silly question, given that soaps are so common. You might know what soap is, and even use it every day, but what about the actual composition?
Also Read: Do Soaps Really Break Down Viruses? If So, How?
Soaps And Detergents
Soaps are obtained from animal oils, fats and strong alkali solutions. Chemically speaking, these are potassium or sodium fatty acids salts produced from the hydrolysis (adding of water molecules) of fats through a procedure called saponification.
A detergent, on the other hand, is a chemical that you use to remove grime and grease. More technically, it is a mixture of ‘surfactants’ (more about them in the next section) that possess cleaning properties in diluted solutions. It’s interesting to note that washing powders are not the only situations where detergents are used. Hair shampoo, stain removers, shaving foam and many others all contain some amount of ‘detergent content’. In simple terms, soap is a ‘type’ of detergent.
Also Read: What’s The Difference Between Various Types Of Soaps, Shampoo And Detergents?
How Does Soap Help Us Wash Clothes?
Now for the big question: how does soap remove grime and dirt from our clothes?
Surface Tension
Water molecules have a tendency to stick together, as opposed to losing their fellow water molecules’ company and sticking to other surfaces. This happens as a result of something known as the ‘surface tension’ of water. Surface tension is the reason why water droplets are spherical in shape, as a sphere is the shape that minimizes surface tension among water molecules.
In order to make water molecules spread/diffuse on a given surface, you have to reduce their surface tension. But how is it done?

This is where surfactants enter the picture. Surfactants are substances that help lower the surface tension of water molecules, thus ‘persuading’ them to wet things more uniformly.
Now, can you guess where you might find a decent amount of surfactants?
Soaps, of course! Soaps (which are a type of detergent) contain surfactants in significant amounts, which help water spread uniformly over clothes. However, this is only one thing about soaps that help clean clothes; the other is…
The Chemical Structure Of Soap
Take a look at the chemical structure of soap in the following image:
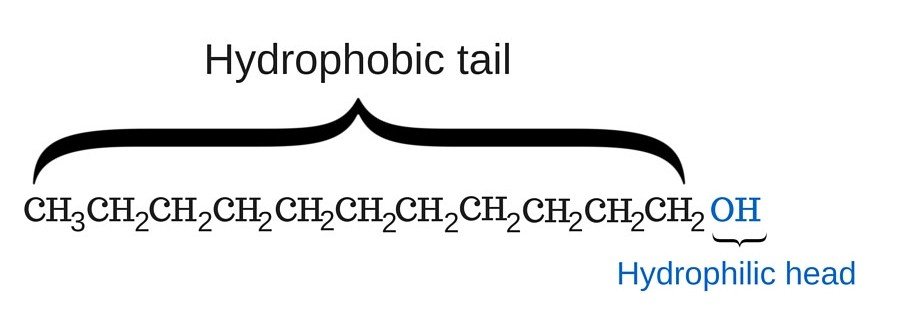
As you can see, one end (the head) of the soap molecule is hydrophilic; this part has a strong affinity for water molecules (in other words, it loves water). The other end (the tail) of the soap is hydrophobic; this part tries to stay away from water molecules, as it has a strong aversion to it. However, it does love dirt and grime. These two parts of a soap molecule, given that they have a contrasting disposition towards water molecules, is why soap is so effective for washing clothes.
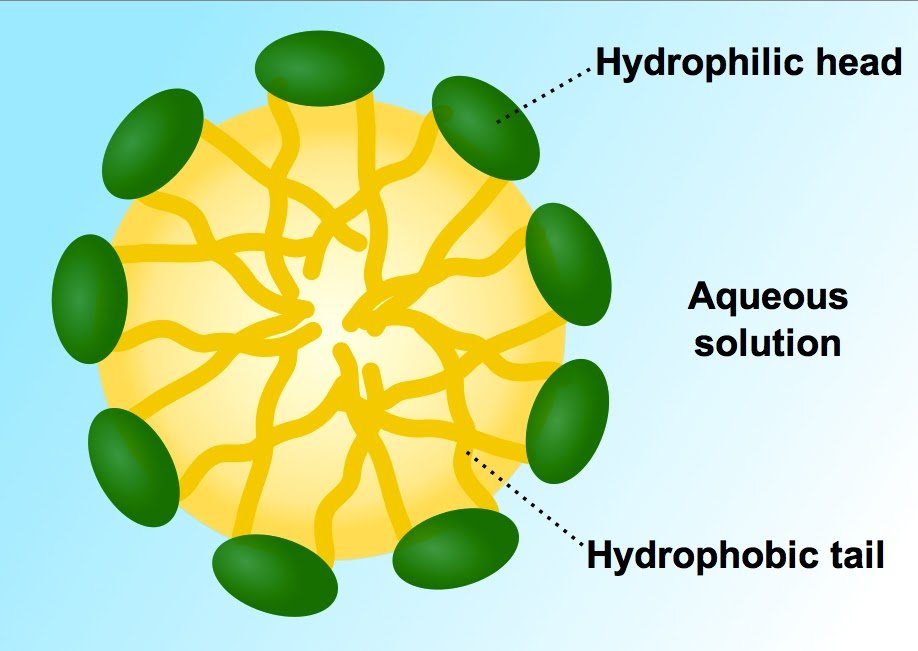
The hydrophobic tail attaches itself to grime and dirt, while the hydrophilic head gets affixed to water molecules. Therefore, when the dirty clothes are put inside a washing machine or swished vigorously by hand, dirt is pulled away from the cloth and washed away in the water, leaving the cloth sparkling clean.
It turns out that a mixed relationship with water, in some cases, can be a good thing. It’s more beneficial for some, particularly the entire washing machine and detergent industries, which cash in big on soap’s loving-and-loathing attitude towards water!
How well do you understand the article above!

