Unlike charges are attracted to each other, but protons and electrons within the space of an atom do not interact with each other. Quantum physics attempts to explain the reason behind the absence of this forbidden interaction.
The foundation of the question ” Why do electrons not collide with protons?” comes from the Rutherford planetary model of an atom.
This is an overly simplified model of an atom as a central dense nucleus consisting of protons and neutrons, with electrons revolving around the nucleus, an idea structured on the solar system. Quantum physics attempts to explain this interaction less abstractly.
Rutherford Model As An Extension Of The Solar System
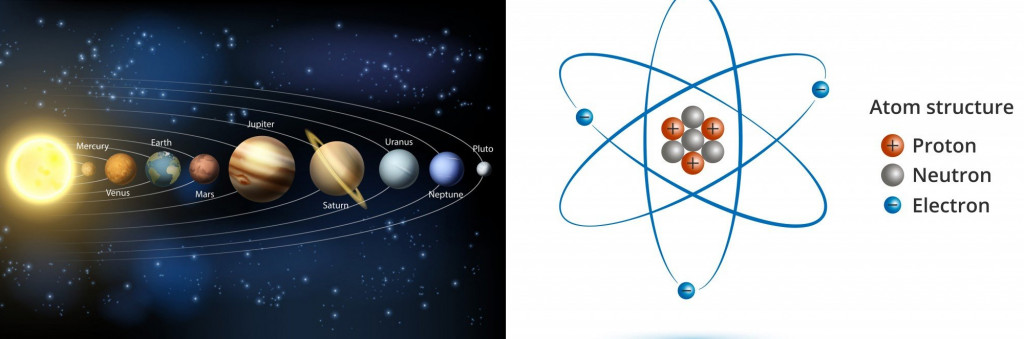
This model failed to explain the stability of a particle in a circular path, but it did leave an indelible question that has lingered for generations: Why don’t electrons end up pulling on their protons?
Rutherford hypothesized that the stability of an electron is a balance between the centrifugal force of the revolving electron and the attractive forces of the nucleus. It is a perfect picture hypothesis, but unsustainable!
Why Is Rutherford’s Planetary Model Invalid?
A charged particle revolving in an orbit must change direction, resulting in acceleration. A charged accelerating particle will lose energy in electromagnetic radiation, and eventually collapse into the nucleus.
However, this does not happen. An atom is extremely stable, so we need to move away from classical physics and spin towards quantum physics for an answer.
Also Read: What Is Bohr’s Atomic Theory?
Evolution Of The Structure Of An Atom
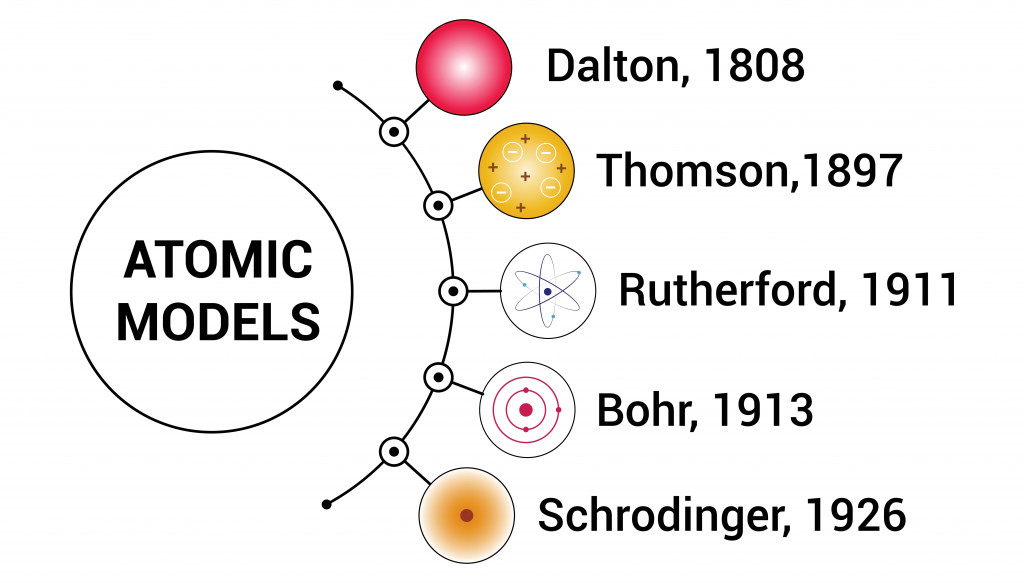
- (1803) Dalton’s billiard ball model of an atom as an indivisible entity. Today, we know that an atom is divisible into sub-atomic particles.
- (1904) Thomson’s plum pudding model embedded electrons in a sphere of positive charges. The theory failed upon the discovery of the nucleus by Rutherford and his team.
- (1911) Rutherford’s nuclear model proposed that the atom has a small, dense, central region, called the nucleus, consisting of positively charged particles. The negatively charged electrons revolved in orbits around the nucleus. This model, based on the planetary model, does not explain the stability of an atom.
- (1913) Niels Bohr’s quantum model was also based on a planetary model, but here, the electrons revolve in paths with fixed energy called orbits. The spaces between the orbits are forbidden for the electron. This model rules out the spiraling of electrons into protons because of the “forbidden paths” between different energy levels.
However, the model could not explain the line spectra of atoms with more than one electron.
- (1926) In Schrodinger’s quantum mechanical model, electrons do not move in circular orbits but exist in electronic clouds. An electron cloud is a region of space inside an atom, where there is a 90% probability of finding the electron; this space is called the orbital.
Schrodinger and Heisenberg put forward theories and mathematical equations for the stability of an atom, which led to the birth of quantum mechanics. According to this school of physics, the position and momentum of an electron cannot be determined simultaneously.
With the electron cloud model of an atom, there is no forbidden zone for electrons to cross.
Also Read: What Is J.J. Thomson’s Plum Pudding Model?
Then What Stops The Electrons And Protons Of An Atom From Interacting?
A simple experiment can show that protons and electrons of different atoms interact, but protons and electrons of the same atom do not interact. Protons and electrons are of opposite charges, so traditionally, they would be attracted to each other.
This is very clear from a small balloon experiment. Static electricity is an electrical phenomenon in which charged particles can be transferred from one body to another.
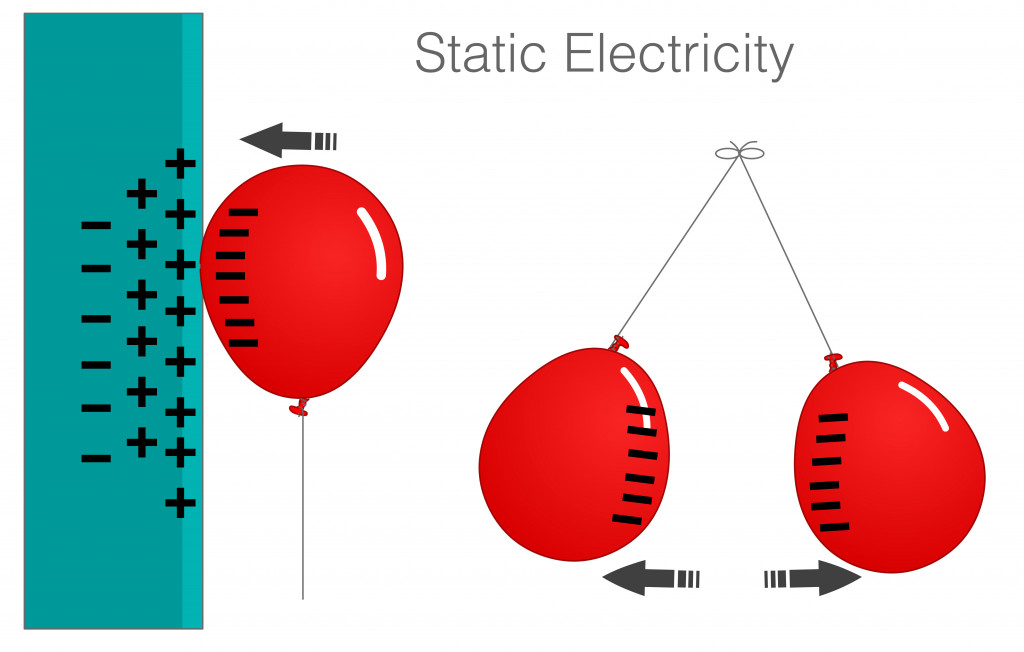
When a balloon is rubbed against a sweater or a person’s hair, the balloon gains negative charges. When the negatively charged balloon is brought close the wall, the electrons in the wall move, leaving the protons exposed, which interact with the negative charges on the balloon.
When the electrons from one type of matter are attracted to the protons of another, then why do electron and protons within the same atom not interact? In theory, electrons should zoom right into the nucleus!
Also Read: Why Do Like Charges Repel And Opposite Charges Attract?
Odds Against Nuclear Collision
Four concepts forbid interactions between protons and electrons of the same atom.
1. Kinetic And Potential Energy In Atomic Stability.
An electron in atomic space farther away from the nucleus carries potential energy, but no kinetic energy. If the electron moves towards the proton, part of its potential energy is converted to kinetic energy and electromagnetic energy. Electrons with kinetic energy keep hopping, which prevents them from combining with a proton.
2. The Final Deck In The Game: The Battle Of The Infinities
If the electron did enter the nucleus, it still wouldn’t combine with the proton. The potential energy of an electron becomes negative as it approaches the nucleus and is minus infinity inside the nucleus. In contrast, the kinetic energy of electrons keeps increasing and it is positive infinity inside the nucleus, which is called the confinement energy. A fall in potential energy is twice the kinetic energy. This will make the electron hop at a distance equal to Bohr’s radius, thus limiting its interaction with protons.
3. Dual Nature Of Electrons
As per the Heisenberg principle, the location and the momentum of an electron cannot be determined simultaneously. This is a fundamental property of microbodies, such as electrons. So, within the perimeter of an atom, an electron cannot be considered as a particle, but more as a wave. Thus, the electron can pass through the nucleus, but it cannot fall and remain in the nucleus.
4. Let’s Sum It Up
A proton–electron union must form a neutron. Both the charge and the mass have to match. Charge-wise, the positively charged proton will interact with the negatively charged electron to form a neutron, but the match of mass is improbable. The mass of a proton is 1.6726 x 10-27 kg, and the mass of an electron is 0.00091 x 10-27 kg, but the mass of a neutron is 1.6749 x 10-27 kg. The sum of the mass of an electron and proton is not equal to the mass of a neutron.
Thus, for an electron and proton to combine together to form a neutron, energy, mass, or both must be added.
Also Read: What Is The Electron Cloud?
Conclusion
Electrons may occasionally enter the nucleus, but it is improbable for them to interact with protons to form a neutron. Several concepts explain the absence of this fatal attraction: the battle of infinities, the wave property of electron, and the gap in the mass of a neutron to the sum of the masses of a proton and electron. The forbidden interaction of protons and electrons is a fundamental property that keeps atoms and the Universe intact!
Also Read: If Weak Force Is So Weak, Why Is It Needed At All?
How well do you understand the article above!

