Table of Contents (click to expand)
There are several ways to remove rust from tools. One way is to use baking powder. Another way is to use vinegar or citric acid. Another way is to use WD-40 or a dedicated rust remover. Another way is to use electrolysis.
Rust is one of the biggest threats that can cause the utility of tools and machines to stoop to the level of dead weight. Like a highly contagious disease, rust gradually spreads over a metal, infecting every atom of which it is composed.
If it can consume a one-ton car whole, what can an 18-pound wrench do to stop it? The onset of rust fatally reduces a tool’s efficiency, which forces you to choose costly maintenance or expensive redesigns. More infuriating is that none of this is your fault!

Before moving onto the tips for how to clean rust, it’s best to understand why iron rusts in the first place to get a better grasp of how these methods resurrect the tools from the brink of uselessness.
Why Do Metals Rust?
Rust is a consequence of a destructive electrochemical attack on metals that is formally known as corrosion. Corrosion occurs when a metal reacts with its environment; more precisely, it is an atmospheric oxidation of metals. Corrosion forms a new layer that, despite its nefarious reputation, isn’t necessarily bad; these layers can also be good, in some instances.
However, in the case of iron, they are ruinous, and because iron and steel are of utmost importance in industrial and household lives, the prevention of corrosion is essential. The corrosion of iron is known as rust. The formation of rust is a reaction between iron, oxygen and water (moisture) to form a new reddish-brown and rugged layer upon it, a layer of hydrated iron oxide and iron oxide-hydroxide.

Some precautions can be taken before exposing a tool to the open air, such as storing them with silica gel packets, painting them, or galvanizing their surface with a sacrificial metal. This prevents the metal from coming into contact with its environment.
However, protection layers are ephemeral; they eventually wear off, permitting the air to attack the metal through crevices. In these cases, there are some DIY and more sophisticated methods to remove the rust and free the colonized iron. More importantly, these methods are backed up by science!
Also Read: What Is Oxidation?
Baking Powder
This remedy can be easily applied at home. Grab some baking soda and mix it with some water to form a thick paste. However, it is imperative to see that the paste is not too thin or too viscous. Furthermore, paint the rust-coated tool with this paste and leave it on for a few hours. Later, remove the rust by scuffing it thoroughly with a brush.

One limitation is that stubborn coatings cannot be easily removed by brushes, as forceful scuffing could scratch or damage the metal. This is also true for sandpapers, something that is often recommended for getting rid of rust.
Vinegar And Citric Acid
Another household remedy is the use of weak acids, such as vinegar or acetic acid, and lemon acid or citric acid. The rusted tool can be submerged in a bottle filled with vinegar, the lid is closed, and the bottle is shaken vigorously. The tool should be allowed to soak for a day to allow the acid to work its magic.
Acids dissolve the rust layer because the iron displaces the pre-existing anion, say, the hydrogen in the acid. However, weak acids are recommended because strong acids not only dissolve the layers of rust but also immediately dissolve the fresh layer of iron beneath it. This method can be used to effectively clean screws and nails.

As for utilizing citric acid, apply salt all over the tool and squeeze a lemon over the tool. Leave it for hours and scrub the tool to remove rust. If the rust remains, repeat the process again and leave it for a day, followed by another scrub. Again, strenuous scrubbing could damage the tool, so be careful.
Also Read: Why Is Stainless Steel Cleaned Using Vinegar And Aluminum Foil?
WD-40 And Dedicated Rust Removers
Discarded tools are the ones that usually acquire rust. Tools are always recommended to be stored in drawers or kits with silica gel packets as a precautionary step, but rust can still break through. Other than using weak acids, you can spray a penetrating lubricant, such as WD-40, and scrub the tool with a Scotch-Brite pad.

For a more severe settlement of rust, use a strong acid based on a verified rust remover, such as Rust Free. Another resourceful product is Evapo-Rust, which restores the appearance of the metal to an almost novel kind!
Your Last Resort? Electrolysis
“Lysis” is a Greek word that translates to “tear apart”, and electrolysis literally means to tear apart with electricity. This method is used to break apart bonds with the help of an electric current. In the case at hand, electricity can be used to break the iron hydroxide bond and remove rust from the iron’s surface.
It requires a large bucket filled with a solution of washing soda and water, which acts as a safe route for electrons on the move and prevent the formation of unsafe products. The tool suffused with rust and a sacrificial metal is dipped into this solution. The positive terminal of a battery (a car battery would do) is connected to the sacrificial metal and the negative terminal is connected to the iron you intend to clean.
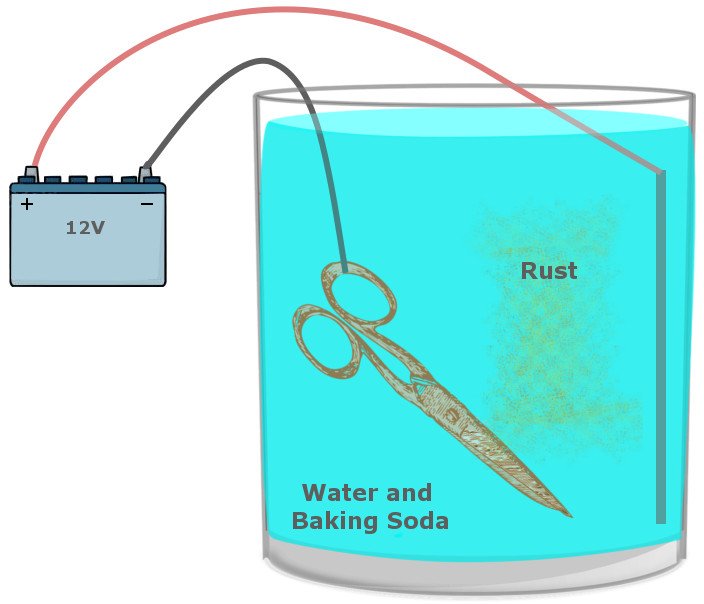
When the battery is powered, the reaction that facilitates rust is reversed, such that the metal now connected to the positive terminal sacrifices itself for the iron and becomes oxidized instead. On the other side, the iron connected to the negative terminal receives electrons and is reduced.
Leave the tool soaking for a day and then remove it the next day to examine whether the rust has been removed. If not, the process can be repeated for another day to ensure that every speck of rust is eradicated.
Also Read: Why Is Rust A Poor Conductor Of Electricity?
How well do you understand the article above!

