Table of Contents (click to expand)
The Haber-Bosch process combines atmospheric nitrogen with hydrogen to produce Ammonia, which is a major component of fertilizers used to promote plant growth.
The Haber-Bosch process, invented in 1909-1910, is one of the most important inventions made in the field of science. The process is named after German chemists and inventors, Fritz Haber & Carl Bosch. The process combines atmospheric nitrogen with hydrogen to create ammonia. Sounds simple, right? Well… it isn’t.
In fact, the invention of the process is of such importance that Haber and Bosch were awarded Nobel Prizes for their work. Now, you may be wondering why the production of Ammonia was considered such an extraordinary feat deserving of the Nobel Prize. Well, before we get into why it’s so important, let’s look at the process itself.
What Is The Haber-Bosch Process?
The Haber-Bosch process converts atmospheric nitrogen (N2) to ammonia (NH3) by combining it with hydrogen (H2). The process combines a single nitrogen molecule with 3 hydrogen molecules to produce 2 molecules of Ammonia. The chemical equation for the Haber-Bosch process is
N2 + 3H2 ⇌ 2NH3
The ⇌ arrow in the above equation implies that the reaction is reversible in nature. The reaction also happens to be exothermic.
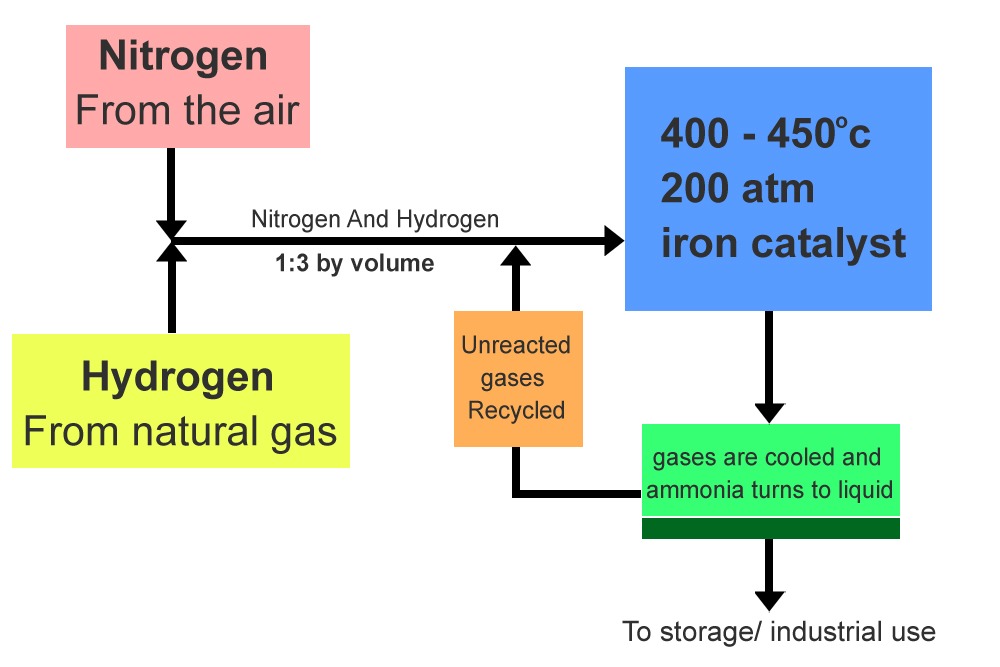
The primary source of hydrogen is methane (natural gas). In fact, around 3% to 5% of the world’s total natural gas production is used in the Haber-Bosch process, while nitrogen is extracted directly from the atmosphere. The reaction occurs in the presence of an iron catalyst, as well as at high temperatures and pressures.
In every pass through the reaction apparatus, only about 15% of the two reactants get converted into nitrogen. The unreacted/uncombined gases are recycled and passed through the apparatus again. The produced ammonia molecules are passed through a condenser for condensation and liquid ammonia is extracted for storage and industrial use.
Role Of The Catalyst & High Pressure
Nitrogen, in the atmosphere, is present in the form of diatomic molecules. The molecules are linked to each other via triple covalent bonds. These bonds are very strong and difficult to break. The catalyst helps break these bonds between nitrogen molecules and also adsorbs nitrogen and hydrogen on its surface.
The original Haber-Bosch process made use of osmium as the catalyst. However, osmium is not readily available. Most modern versions of the Haber-Bosch process use an iron catalyst instead. Iron with promoters like KOH, K2O, Mo, and Al2O3 provide a perfectly porous and high surface area material for the reaction.
The high temperatures and pressures encompassing the chemical reaction help to bypass chemical equilibrium and prevent the reaction from progressing in the reverse direction. A reaction is said to be at chemical equilibrium when the number of reactants and products do not change with time.
At the beginning of the reaction, the amount of nitrogen and hydrogen is greater, so the reaction produces ammonia at a high speed. As the concentration of the two reactants decreases, the conversion rate drops. A point is soon reached when the amount of ammonia being produced and the speed at which ammonia decomposes becomes equal. This state is called chemical equilibrium.
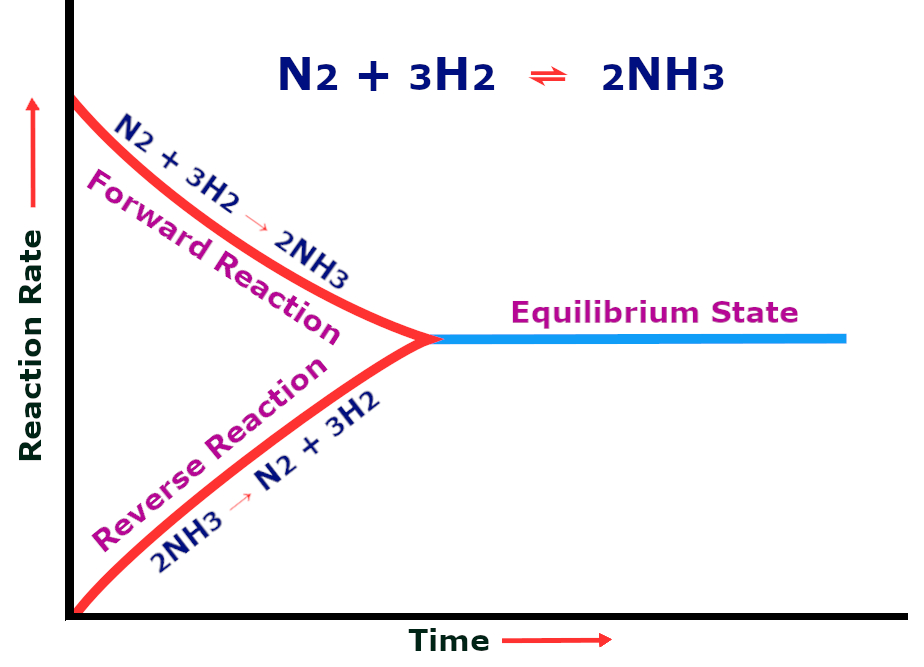
At this point, no more ammonia is being produced. In order to continue the production of ammonia and disrupt the equilibrium, the pressure is increased. According to Le Chatelier’s principle, “If you increase the pressure of a system, it will respond by favoring the reaction that produces the fewer number of molecules and helps decrease pressure”
In the Haber-Bosch process, the combination of nitrogen and hydrogen results in the production of fewer overall molecules. Thus, the reaction restarts in the forward direction and the production of ammonia is resumed.
Also Read: Why Do Explosives Have Nitrogen In Them?
Why Is The Haber -Bosch Process So Important?
The Haber-Bosch process is critical for two reasons: Firstly, the process allows us to use the extensive amount of nitrogen available in the atmosphere. Second, the end product of the process has fed and continues to help feed more than half of the global population. I’ll explain that after we have a look at how plants prepare food and why nitrogen is essential for that process.
Why Do Plants Require Nitrogen?
Plants prepare food through a process called photosynthesis. Chlorophyll, the pigment responsible for the green color of plants, helps them absorb energy from sunlight and perform the photosynthesis reaction. One of the vital components of the chlorophyll compound is nitrogen. Thus, a lack of nitrogen means no photosynthetic reactions. Even though nitrogen is one of the most abundant elements on Earth, nitrogen deficiency is a common occurrence in plants.
The strong covalent bonds between atmospheric nitrogen N2 render the molecules inert and of no use to plants. Since plants cannot use atmospheric nitrogen, they look for nitrogen in the soil beneath. Bacteria and Archaea present in the soil convert N2 molecules into Ammonia (NH3) and various nitrogen oxides. Breaking down atmospheric nitrogen and converting it to a form that is useful to plants is called Nitrogen Fixation. For more information on Nitrogen Fixation, read: What is the Nitrogen Cycle?.
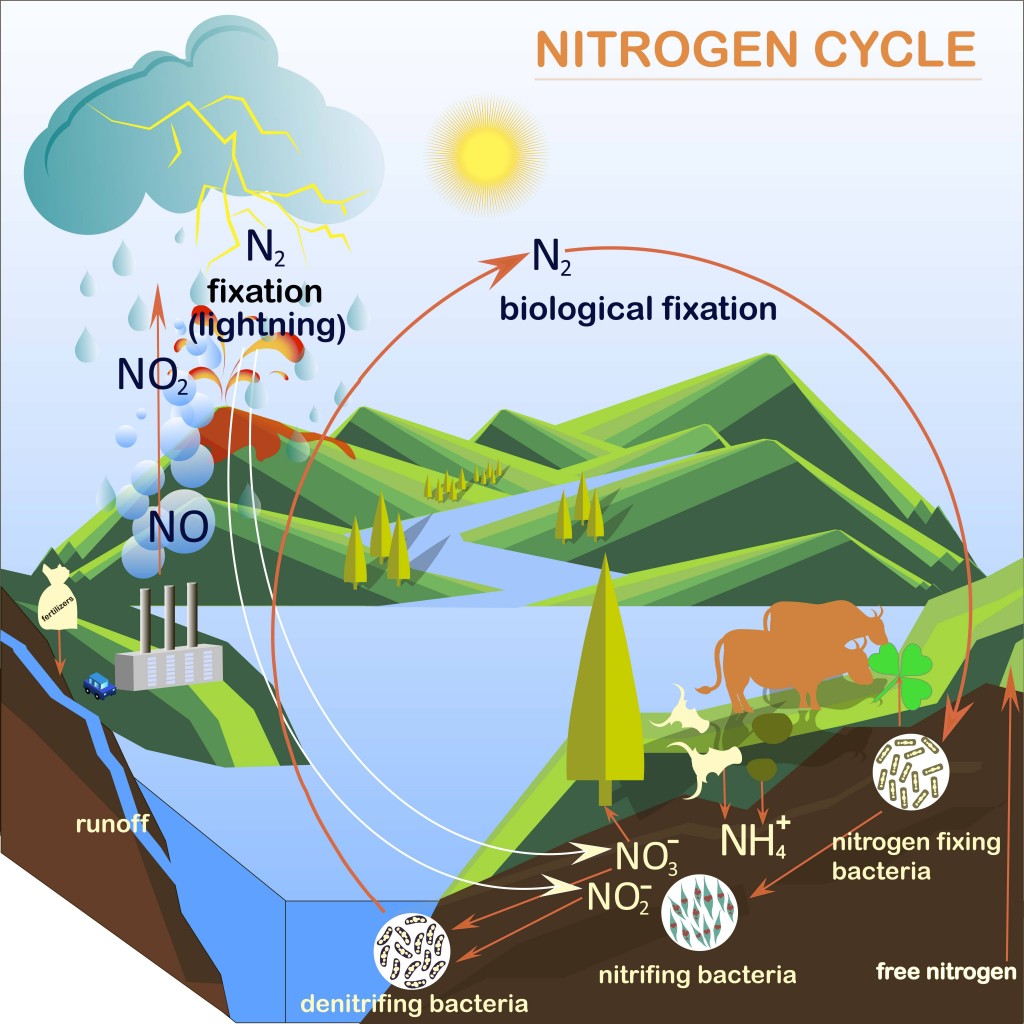
The amount of nitrogen that is naturally fixed is very low, and the process is very slow. Farmers in the 1900s therefore had to employ agriculture techniques like crop rotation or use cow dung as fertilizer to be able to grow plants at a decent rate.
Impact Of The Haber-Bosch Process On Food Production
The development of the Haber-Bosch process helped us convert nitrogen into forms that are far more useful to mankind in larger quantities and with greater speed. Thus, synthetic fertilizers containing ammonia could be easily produced and used to boost plant growth.
The development and use of synthetic fertilizers have played a significant role in sustaining population growth over the last century. The global population, in fact, has quadrupled since the discovery of this process. The global population in the early 1900s was approximately 1.7 billion, while the current global population stands at a whopping 7.7 billion. Without fertilizers, only about one-third of the food currently produced would be available.
Also Read: How Do Fertilizers Harm The Environment?
Conclusion
Considering the impact of fertilizers on food production, it wouldn’t be an overstatement to say that the Haber-Bosch process has helped feed more than half of the global population. Currently, about 400-450 million tons of nitrogen-based fertilizers are manufactured every year using the Haber-Bosch process.
However, fertilizers are only one of the products derived from ammonia. Other uses of ammonia include its use as a refrigerant, in the production of explosives, nylon/plastic production and much more.
Still curious why Haber and Bosch deserved Nobel Prizes for inventing the process? Try putting food on the plates of half the global population and you might win one too!
How well do you understand the article above!

References (click to expand)
- How do plants get their nitrogen from the air?. West Texas A&M University
- Zmaczynski - Haber Process - Princeton University. Princeton University
- The Haber-Bosch Process - nitrogen reduction. The College of Saint Benedict
- How a century of ammonia synthesis changed the world - www.physics.ohio-state.edu
- Carbon_Dioxide - mattson.creighton.edu
