Table of Contents (click to expand)
To keep a cup warm, heat losses through conduction, convection and radiation should be minimized as much as possible. The easiest way to do this is to hold it in your hands and cover the top.
The month is January and winter is in full swing. It’s snowing outside, but you have to reach the office. Your hands are freezing and you grab a cup of warm coffee to beat the chill. Holding the cup in your freezing palms, your hands slowly warm up and become comfortable again. However, you notice that the cup has become colder and wonder if putting the cup on the table would have kept it warmer.
But would the coffee have stayed warmer if placed on the table? And why did the cup cool down in your hands anyway?

The answer to the above questions is provided by Thermodynamics.
Heat Energy And Heat Flow
Thermodynamics (thermal + dynamic) is a branch of science that studies the interaction of heat energy with the surrounding matter. The feeling of hot or cold is a measure of kinetic energy (energy due to motion) of the molecules constituting that object. If something is perceived as being hot (a higher temperature), then the molecules of that object possess greater kinetic energy than an object that is perceived to be cold (lower temperature).
Whenever two objects at different temperatures (i.e., constituent molecules having different kinetic energies) are brought in contact with each other, the following happens:
- The object at a higher temperature (higher kinetic energy of molecules) loses heat.
- The object at a lower temperature (lower kinetic energy of molecules) gains heat.
- The flow of heat from the object at a higher temperature to the object at lower temperature occurs as long as a temperature differential exists. The flow stops when both the objects reach the same temperature. This is called ‘thermal equilibrium’.
Mechanisms Of Heat Transfer
Heat transfer occurs in the following ways:
1) Conduction: The flow of energy between two objects at different temperatures in physical contact with each other is called conduction. Kinetic energy is transferred due to the collision of molecules at the interface of both the objects without inter-mixing, e.g., your palms wrapped around a warm coffee cup.
The molecules of coffee, having high kinetic energy, collide with the cup walls and impart energy to its molecules. Some of that kinetic energy is then imparted by the sides of the cup to your hands.
The rate of heat transferred per unit time (Q) is given by:
where
A = Area of surface (cup wall) in contact between the hotter object;
k = Thermal Conductivity of the medium (cup) through which heat flows;
d = Thickness of medium (cup cross section) conducting heat;
 = Temperature of hotter object (coffee);
= Temperature of hotter object (coffee);
 = Temperature of colder object (Palms);
= Temperature of colder object (Palms);

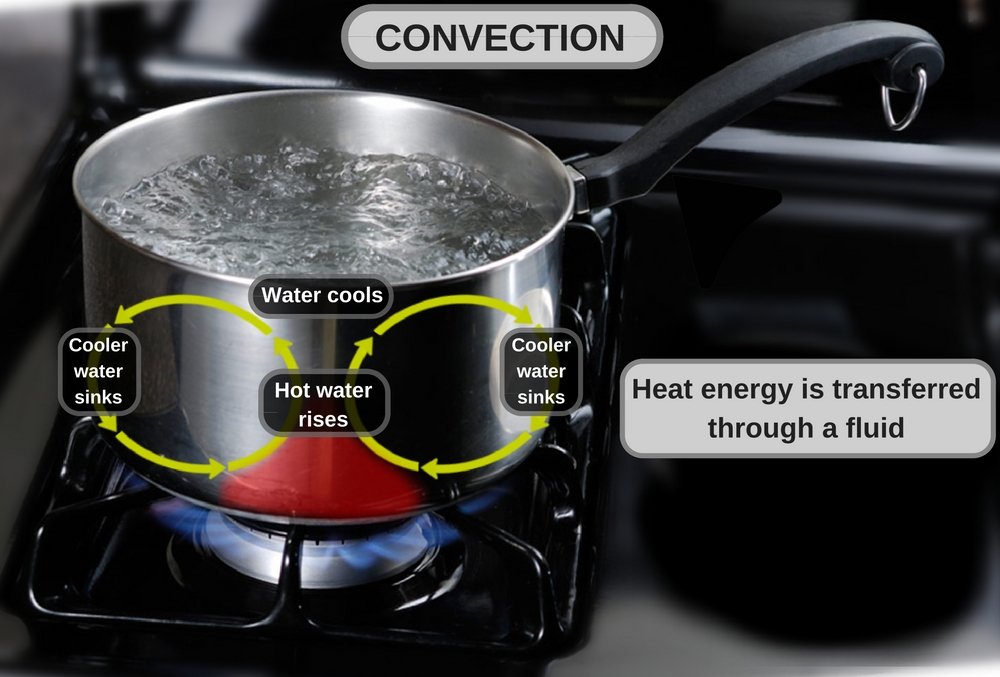
2) Convection: The flow of energy from a fluid to a surface due to the movement of fluid in bulk along the surface is called convection. Transfer of heat occurs from the fluid to the surface, e.g., heat felt on the palms beside the hot coffee cup (without your hands touching the cup).
The cup walls gain energy by conduction from coffee. The walls then heat the surrounding air through conduction. The hot air beside the cup rises and moves along the surface of the nearby palms, imparting energy. The rate of heat transferred per unit time (Q) is given by:
where
h = Convective heat transfer coefficient;
A = Area of the surface in contact with fluid;
 = Temperature of the fluid in motion;
= Temperature of the fluid in motion;
 = Temperature of the surface;
= Temperature of the surface;
3) Radiation: The flow of energy in the form of electromagnetic waves without the presence of a physical medium is called radiation. If a medium is present, no energy is imparted to that medium (unlike in conduction and convection).
Every object in the universe radiates energy in the form of electromagnetic waves at the infrared end of the EM spectrum. A cup also radiates EM waves. The open top imparts direct radiation from the coffee. The closed bottom and sides gain heat energy by conduction from the coffee, and then radiate some of that energy to the surroundings. The amount of heat energy radiated per unit (Q) time is given by:
where
 = Emissivity of the object (gives an idea about how good an emitter the object is);
= Emissivity of the object (gives an idea about how good an emitter the object is);
A = Area of radiating surface (Open top + Lateral Area of Cup walls);
 = Stefan’s Constant
= Stefan’s Constant
 = Temperature of radiator
= Temperature of radiator
 = Temperature of surroundings
= Temperature of surroundings
Also Read: If Heat Cannot Travel Through A Vacuum, Why Does The Sun Feel Hot?
The Answer
The cup of coffee in our case loses heat through the three mechanisms discussed above. The question presents the following scenarios:
| CASE 1: Cup Kept On The Table | CASE 2: Cup Held In Palms |
| The open top (coffee) radiates heat to the surroundings, given by mechanism 3 | The open top (coffee) radiates heat to the surroundings, given by mechanism 3 |
| The bottom surface loses heat through conduction to the table, given by mechanism 1 | The bottom surface radiates heat to the surroundings, given by mechanism 3 |
| The sides of the cup radiate heat to the surroundings, given by mechanism 3 | The sides of the cup conduct heat to the palms, governed by mechanism 1 |
(NOTE: Since convection is the transfer of heat due to the bulk motion of fluid along a surface, it doesn’t contribute to the cases discussed above. If the palms were kept beside the cup, but not touching it, then convection would have contributed.)
When all variables remain unchanged, radiation is the fastest mode of heat transfer (faster than convection and conduction), as it occurs at the speed of light (3 m/s).
m/s).
From the above discussion, it is evident that in CASE 1, there is maximum radiative loss directly from coffee/cup walls. This results in a significantly faster loss of heat than in CASE 2.
Therefore, to keep the cup warm, hold it between your hands!
It can be kept warm for longer if the top lid is closed and the bottom surface is covered to minimize conductive loss. Creating a vacuum layer around the cup would take care of convection losses.
Also, since EM waves obey the laws of reflection, keep a highly reflecting surface (mirror) all around the cup beyond the vacuum layer. The radiation emanating from the cup will be reflected back into the cup, keeping the coffee warm for hours. Or you can save yourself a lot of time and just buy yourself a high-end thermos!
Also Read: Dewar Flask: How Does A Thermos Flask Keep Hot Things Hot And Cold Things Cold?
How well do you understand the article above!

