Table of Contents (click to expand)
Water is a polar molecule because the electronegativity of oxygen is higher than that of hydrogen, meaning that the electron density is shifted towards the oxygen atom in the molecule.
Water is a polar molecule because its oxygen is strongly electronegative and, as such, pulls the electron pair towards itself (away from the two hydrogen atoms), thus acquiring a slightly negative charge.
The polarity of a molecule depends not only on its constituent atoms, but also on how they are arranged around the central atom, i.e. the spatial arrangement of those atoms. To understand this better, let’s discuss the topic in more detail.
What Makes A Molecule Polar?
The polarity of a molecule is related to the shifting of electrons in a particular direction. This, in turn, depends on the polarity of the bonds present in the molecule, as these bonds also contain electrons.
Within a molecule, the atom with the higher power to attract electrons towards itself (i.e., it’s more electronegative than the other atom) will acquire a slight negative charge on itself, and the bond between the two atoms will become polar.
All in all, you could say that the electron density of a polar bond accumulates towards one end of the bond, which results in that end possessing a slight negative charge, while the other end has a slight positive charge. This makes a molecule polar.
Likewise, if a molecule does not have regions of positive and negative charge, it’s considered nonpolar.
However, an interesting thing to note is that the larger the electronegativity difference, the more polar the bond will be within a molecule. Carbonyl compounds are polar because the carbonyl carbon is slightly positive. Thus, shouldn’t carbon dioxide, which contains a positive carbon and two partially negative oxygens, be polar?
Well, carbon dioxide consists of two oxygen atoms attached to a carbon atom. Oxygen atoms are far more electronegative than carbon atoms, and as such, they should hold a partially negative charge, while the carbon atom should be slightly positively charged. However, interestingly enough, that doesn’t happen.
Also Read: Is Carbon Dioxide (CO2) Polar Or Nonpolar?
Take A Look At The Structural Formula Of Carbon Dioxide:
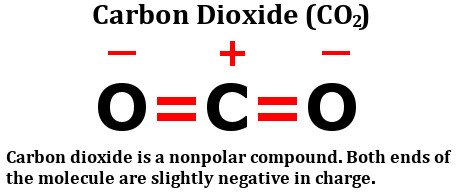
It consists of two equally electronegative oxygen atoms, yes, but look at how these atoms are arranged around the carbon atom. They both stand at perfect 180-degree angles from carbon. Consequently, they pull the electron density from carbon with equal force in opposite directions. The net result is that the electron density on the carbon atom remains unaffected, which renders the carbon dioxide molecule nonpolar.
Carbon dioxide is a great example of how the geometry of a molecule plays a crucial role in determining whether it’s polar or nonpolar. Now, let’s take a look at a molecule of water:
Also Read: What Are The Bubbles Made Of When Water Boils?
Why Is Water Polar?
The chemical formula of water is H20, which means that it contains two hydrogen atoms and one oxygen atom. The hydrogen atoms only consist of one electron in their shell, whereas the oxygen atom has 6 valence electrons.
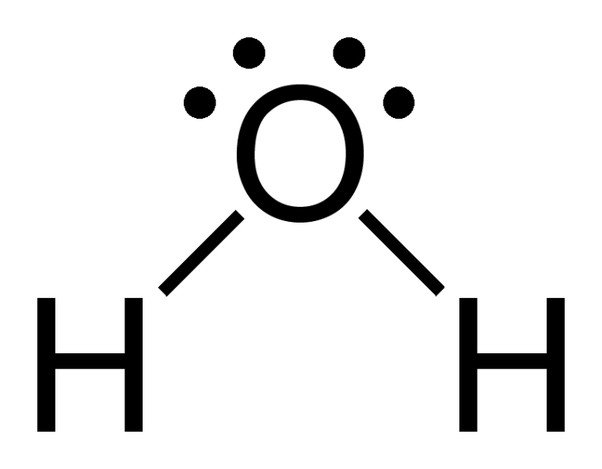
Since oxygen has 6 electrons in its valence shell, it shares an electron with each hydrogen atom. In this way, it’s left with 4 unbonded electrons in its 2 orbitals. These bonded and unbonded electron pairs arrange themselves in a tetrahedral shape around oxygen, which is why the two bonds appear to have a bent shape.
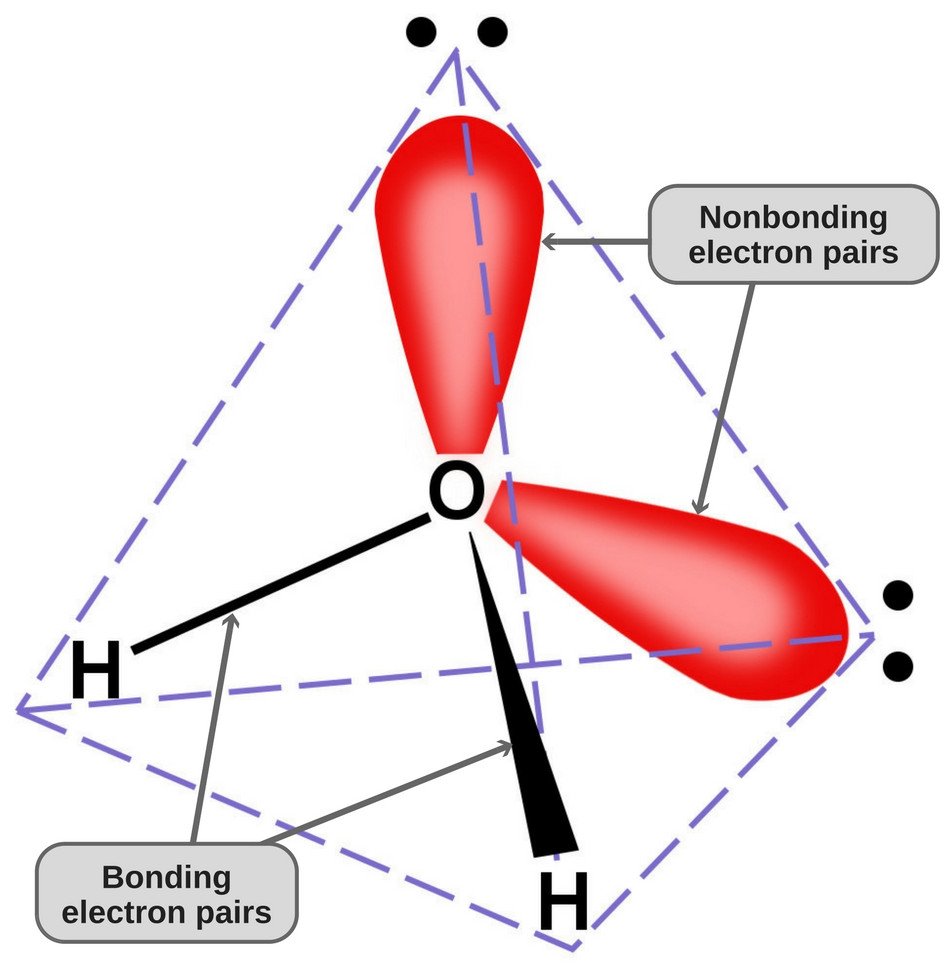
Now, both oxygen and hydrogen atoms have different electronegativities (the electronegativity value of hydrogen is 2.1, while the electronegativity of oxygen is 3.5); therefore, both bonds are polar. Since oxygen is more electronegative than hydrogen, the electron density shifts towards oxygen in both of these bonds, thereby making the region around the oxygen more negative than the areas around the two hydrogen atoms.
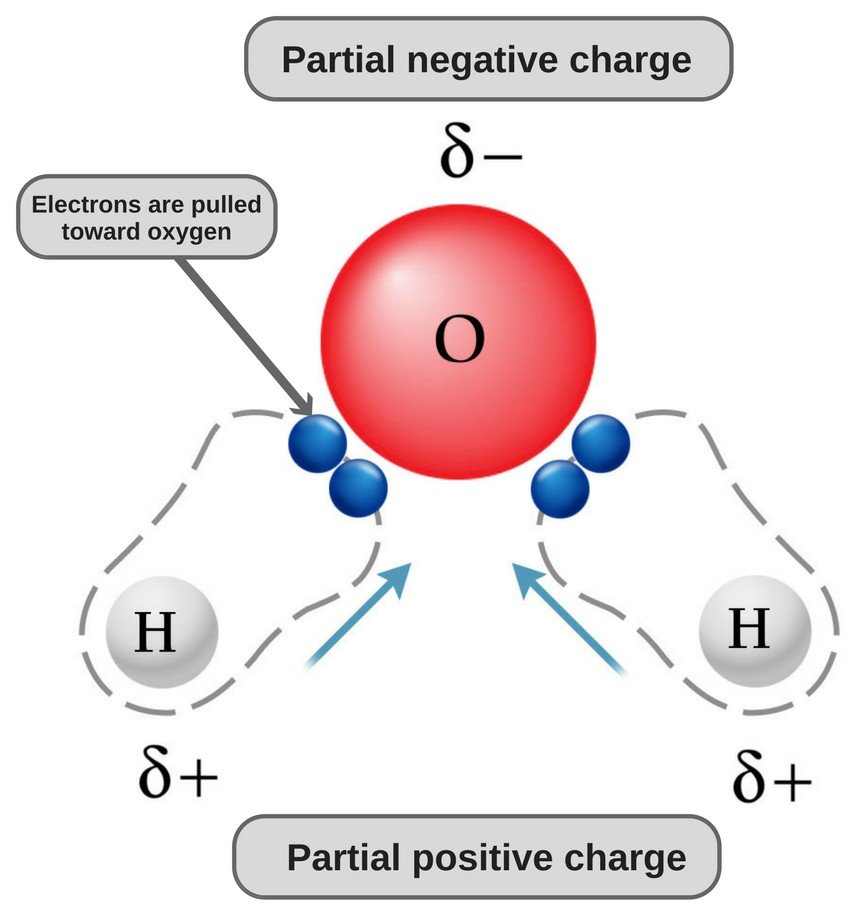
This is why the water molecule becomes polar!
How well do you understand the article above!

