Table of Contents (click to expand)
Esters (R-COOR’) are pleasant-smelling organic compounds formed by a substitution reaction between carboxylic acids & alcohols. They are naturally found in fruits and are commercially used in the manufacturing of soaps, perfumes, polyesters, medicines, etc.
Go ahead and flip over the bottle of perfume you spritz with every morning. Can you find an ingredient with the suffix -ate?
As a matter of fact, if you check the ingredient labels of many other day-to-day products like soaps, detergents, cosmetics, and essential oils, you will find words with the same suffix. Each of these products has at least one awkwardly named chemical ending with -ate. If fruits like pineapples, strawberries, and bananas came with an ingredient label, you would find these chemical compounds in them too!
The -ate chemicals in these everyday products are formally known as esters (R-COOR’) and are a type of organic compound. Generally formed from the union of two other organic compounds, esters are universal in nature; from nanoscopic DNA molecules to giant plastics, they can be found everywhere.
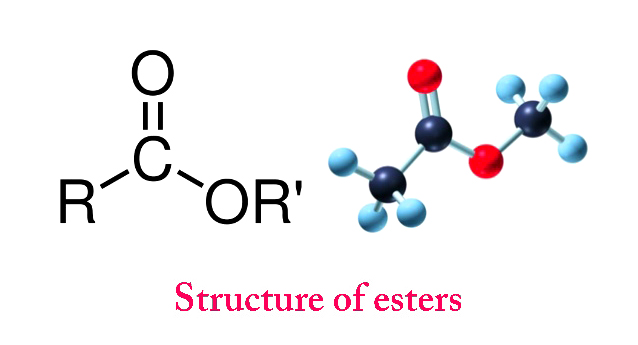
Formation Of Esters
Esters are usually formed by a condensation reaction between alcohol (R’-OH) and carboxylic acid (R-COOH), and the reaction as a whole is known as esterification. Condensation reactions are characterized by the linking of two reactants to form a larger end product (in terms of the number of molecules) and the removal of a smaller molecule. In esterification reactions, water (H2O) is the smaller molecule that gets eliminated.
R’OH + RCOOH ⇌ RCOOR′ + H2O
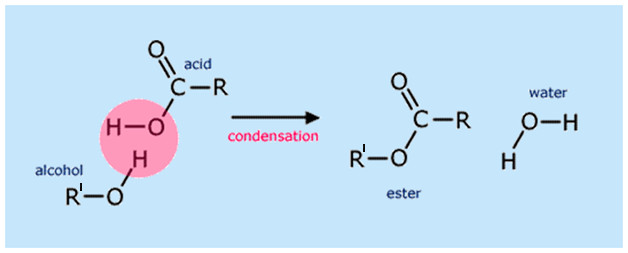
However, organic alcohol and carboxylic acids do not react readily, and when they do, the reaction is highly reversible. Therefore, an acid catalyst, such as sulfuric acid, is generally added to the reactant mixture (Fischer esterification).
The catalyst serves two purposes—speeding up the reaction (like any other catalyst) and acting as a dehydrating agent. Sulfuric acid consumes the eliminated water molecule and prevents the hydrolysis of the newly formed ester. This forces the reaction equilibrium to the right and results in a greater product yield. Additionally, esterification is carried out at temperatures near the boiling point of the reacting alcohol in order to boost reaction rate.
Of course, esterification isn’t the only method used to produce esters. Esters can also be produced by merging two carboxylic acid molecules via treatment with diazomethane or epoxides, reacting acyl chlorides (RCOCl) or acid anhydrides (R(CO)O(CO)R’) with alcohols, or alkenes (CnH2n) with metal carbonyls, etc. An ester can also be derived from another ester using the process of transesterification.
Naming Convention
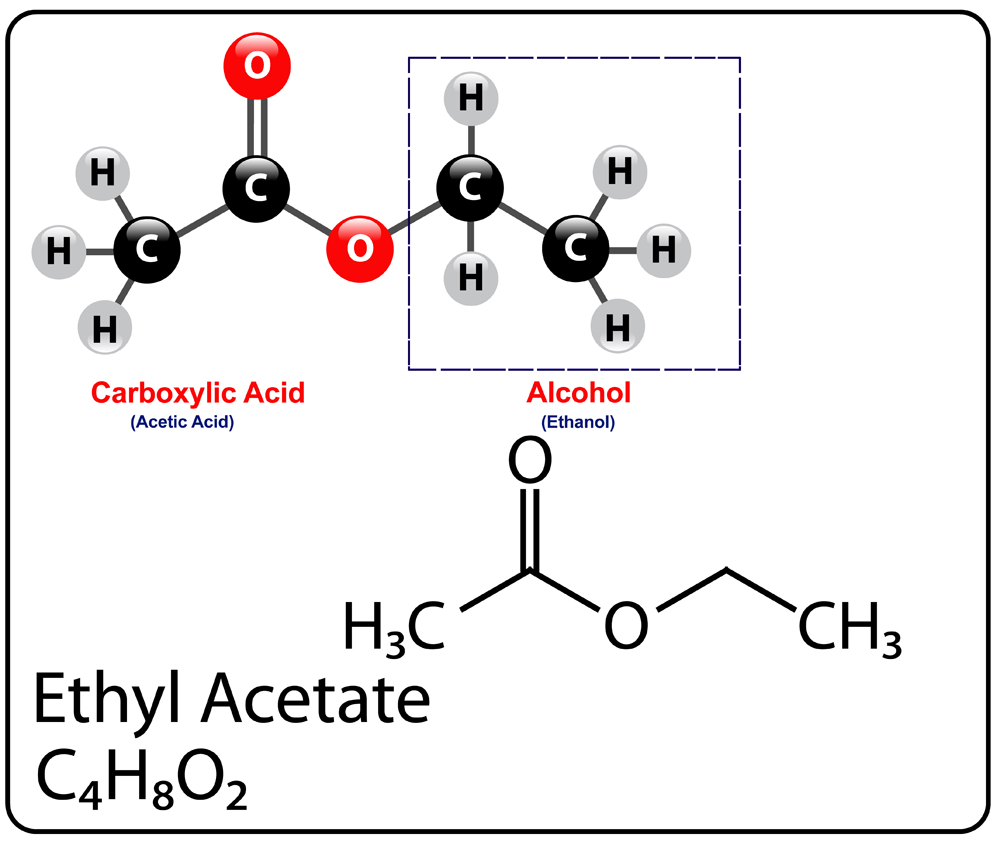
As seen earlier, ester names end with the suffix -ate or -oate and generally contain two words. The first one indicates the attached alkyl group (R’) and the second one is the name of the base carboxylic acid (RCOO-), although, the -ic acid is replaced with -ate.
For example: Ethyl acetate gets its name from the ethyl (C2H5) alkyl group of the parent ethanol, while acetate is obtained by replacing the -ic acid of the parent acetic acid with -ate.
Also Read: What Are Monomers And Polymers?
Physical & Chemical Properties
The physical & chemical properties of esters are influenced by the presence of the carbonyl group (C=O) and the length of the hydrocarbon chain (R & R’) on either side of the carbonyl group; they greatly vary from their parent alcohol & carboxylic acid due to a lack of the hydroxyl group (-OH).
While the carbonyl group grants some polarity to esters, the surrounding hydrocarbon chain is non-polar in nature and renders them only slightly soluble in water. The carbon and hydrogen chain also influences the physical state of esters; the ones with fewer carbon atoms are liquid at room temperature, while those with a longer carbon chain are solid. On the visual and olfactory side of things, esters are colorless and have a rather fruity smell.
Esters have low melting and boiling points (and are therefore extremely volatile), as the hydroxyl group of the constituent alcohol and carboxylic acid is no longer available to form hydrogen bonds with water and other molecules.
As mentioned earlier, the esterification reaction is reversible in nature, and the reverse reaction is called hydrolysis of esters. However, depending on the condition surrounding the reactant mixture, different end products are obtained.
If the conditions are acidic, the ester will break down into its parent alcohol and carboxylic acid, and under basic conditions, carboxylate salts (soap) are produced. This reaction of an ester with a base is formally known as saponification and is the most important reaction that esters undergo. To learn more about the process of soap manufacturing, read – Saponification: How are soaps made?
Uses
Naturally, esters are responsible for the specific odor and taste of a number of fruits and alcoholic beverages. The characteristic fruity smell of esters has been put to commercial use in the manufacturing of perfumes and other personal care products like nail polish remover, lotions, creams, conditioners, etc. and surfactants like soaps and detergents. Esters of para-hydroxybenzoic acid (parabens) are used extensively as food and drug preservatives, owing to their antibacterial and anti-fungal properties.
Ironically, the world’s most bitter-tasting chemical is an ester called denatonium benzoate (Bitrex) and is added to products such as cleaning fluids, fertilizers, and hair dyes to deter people from consuming them.
Personal care products aside, some esters also have medicinal uses. Aspirin, a very important anti-inflammatory drug, is an ester scientifically known as acetylsalicylic acid and is derived from salicylic acid. Methylphenidate, also known as Ritalin, is used in the treatment of ADHD. Certain bad-tasting drugs are often converted to their ester counterparts to negate the harsh flavor.
Esters are equally important on the biological front, as phosphoesters are the backbone of nucleic acids (DNA & RNA), while glycerides—fat storage molecules in animals—are esters of glycerol. Natural esters are also found in pheromones. Industrially, esters hold great value for their interlocking capabilities (polyesters) and the ability to act as a very effective organic solvent. Polyesters are some of the most useful polymers known to man, and find applications in food packaging, clothing, and so much more.
With a myriad of natural and commercial applications, designating esters as “just another organic compound” would be nothing short of criminal!
Also Read: How Do Artificial Flavors Work?
How well do you understand the article above!

