Table of Contents (click to expand)
A monomer is the starting unit for a polymer. It is a single molecule that can react with other monomers to form a polymer through the process of polymerization.
A monomer, as the name suggests, is a single molecule that can react with other monomer molecules to form a polymer. It is the basic unit of a polymer, and can be thought of as the fundamental building block of a polymer compound. That makes it rather simple to define a polymer… it is a large molecule composed of small subunits called monomers. A polymer may also be referred to as a macromolecule. A molecule is nothing but a group of two or more atoms held together by chemical bonds.
Commonly found polymers include carbohydrates, lipids or proteins, and are all made of repeating monomer units. Carbohydrate monomers called monosaccharides are composed of units of glucose and fructose. Lipids are similarly made of fatty acids and glycerol. The DNA or RNA in our body finds its origin from nucleotides, which are monomers. Finally, the building blocks of our body, proteins, are also made of monomer units called amino acids.
It is safe to say that for a molecule to be a monomer, it must have the ability to interact with other monomeric molecules.
What Is A Monomer?
A monomer is a low molecular weight hydrocarbon molecule. In chemistry, a hydrocarbon is any compound entirely composed of hydrogen and carbon molecules. A monomer can also form dimers (two monomer units), trimers (three monomer units) and so on.
Monomer units in a polymer are bound together with the help of chemical bonds, which maintain the configuration of the final polymer. There are various types of configurations that a polymer can have, depending on the monomer units that are bound together.
It is interesting to note that many monomer units have the same number of carbon atoms, but different configurations. Such molecules with same number of carbon atoms, but varying configurations, are called isomers. Glucose, galactose and fructose are common examples of isomeric monomers. They have similar chemical formulas, i.e., similar number of atoms in the molecule, but the molecular arrangement of these atoms is different, giving rise to 3 different compounds.
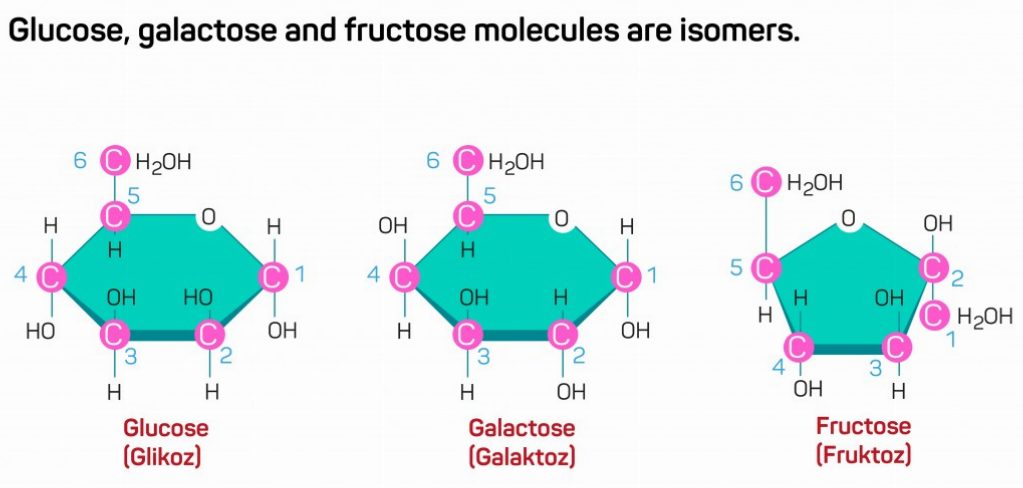
Different isomeric molecules of the same monomer unit confer different properties on the polymeric compound.
Also Read: What Are Diatomic Molecules?
What Is A Polymer?
A polymer is composed of repeating monomer units and can either be natural or synthetic. Polymers form an important part of our system, as mentioned above. Polymers are also found in diamonds, quartz and other man-made materials, such as concrete, glass, paper, plastics and rubber.
A polymer can be a homopolymer or a heteropolymer. A homopolymer has repeating units of the same monomer, such as polyvinylchloride. A heteropolymer has two or more different monomer units. Proteins are a commonly found heteropolymer, due to the variety of amino acids bonded together.
Repeating molecular units are joined together by covalent bonds, which involves the sharing of electrons between the two atoms. This makes the bond relatively strong, as compared to other types of chemical bonds. As a result, a polymer is stronger and much harder to break. The hard shell of a crab is composed of the polymer chitin, which makes it very difficult to break the shell of that creature!
Organic polymers are found in living things and play a very important role, such as the cellulose and lignin in plants. Lignin helps form plant cell walls, whereas cellulose is required to give rigidity to those same cell walls.
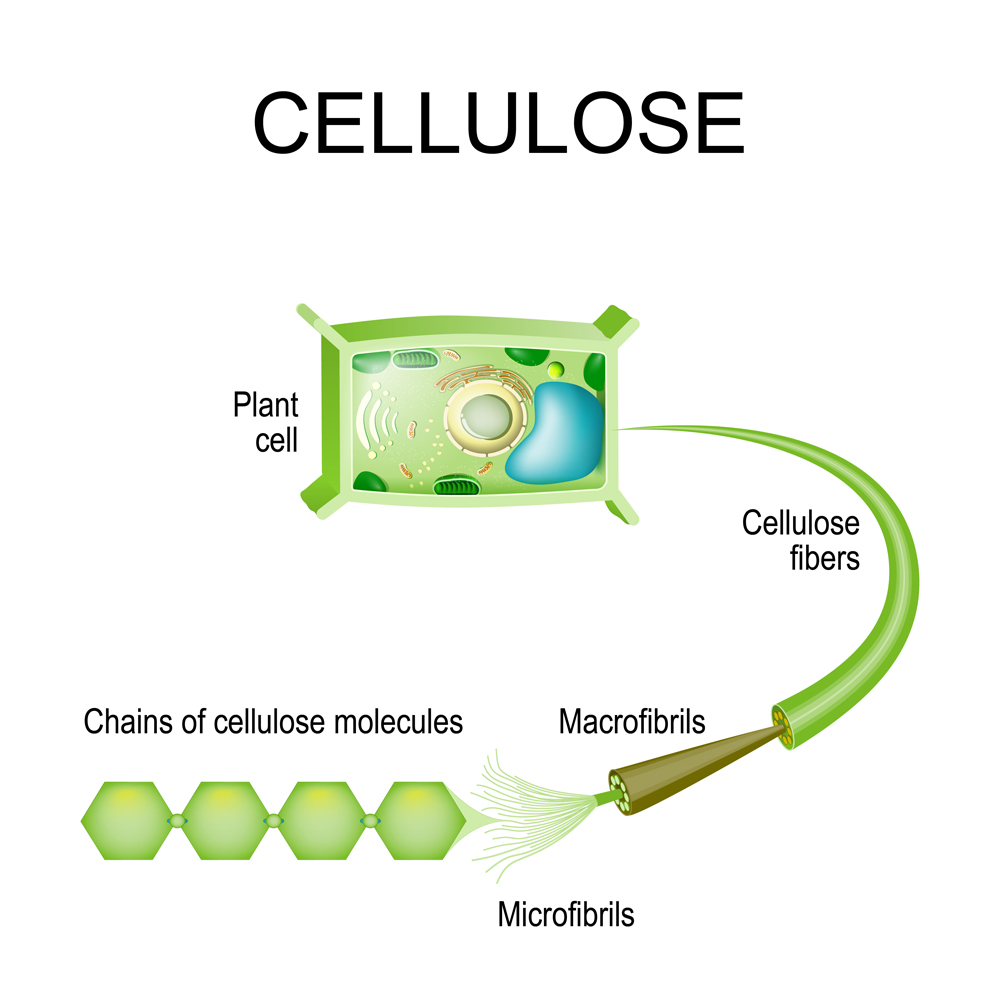
So… how do monomers form these long-chain polymers? It happens by a sophisticated process called polymerization.
Also Read: What Is A Phosphodiester Bond?
What Is Polymerization?
The process of polymer formation through a chemical reaction is called polymerization. During this process, some chemical groups get lost, which leads to a different end product than what was expected. As a result, the polymer does not always retain all the same chemical properties of the original single monomer units. Polymerization reactions come in two types: chain-reaction and step-reaction.
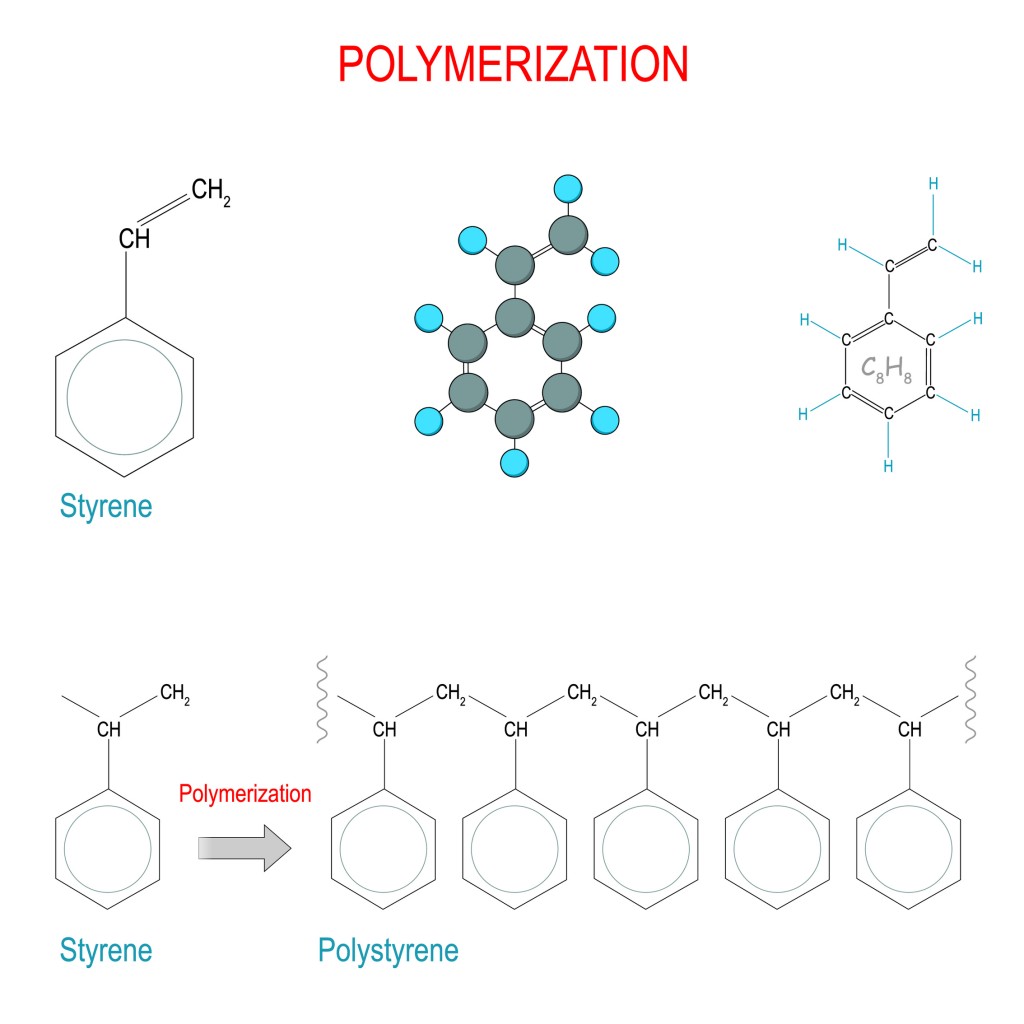
As mentioned earlier, if isomeric monomers are present in the reaction, they tend to affect the way in which the covalent bonding occurs, which modifies the properties of a polymer.
It should be noted that in most cases, a monomer has at least one carbon-to-carbon double bond. A carbon atom has 6 electrons and can form single bonds, double bonds or triple bonds with other atoms. Most of these are covalent bonds and involve the sharing of one, two or three electrons with other atoms, respectively. Atoms bind with each other to gain more stability, so those atoms with an electron to spare are usually more reactive than others.
Any chemical compound with a free electron that can bind to other atoms to form compounds is called a free radical. These free radicals are like catalysts that bind to the double bonds of a monomer unit to form the polymer. The free radical breaks the double bond and binds with the monomer, resulting in the transfer of the free electron from the radical to the outer carbon of the monomer unit. This free electron then sets out in search of other monomer units and a repetitive process begins, wherein monomer units bind to each other. This is the chain-reaction type of polymerization. The polymers produced in this way are high molecular weight compounds.
The step-reaction polymerization, on the other hand, produces low molecular weight compounds and requires a higher temperature. This is also called a condensation reaction, as byproducts like water or hydrochloric acid may be formed during the reaction. Most of these condensation polymerization reactions involve a bi-functional monomer. A monomer with two different functional groups is called a bi-functional monomer.
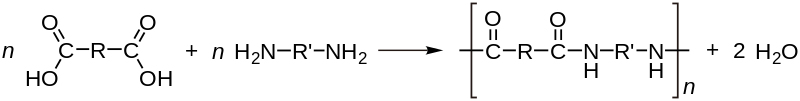
A substituent is an atom or group of atoms that replace one or more hydrogen atoms present on the original hydrocarbon, resulting in a new molecule. This gives us the definition of a functional group as a specific substituent within the molecule that helps confer a different chemical property to the molecule. This bi-functional monomer can then bind with other monomers in both directions and allow the chain to grow, thus giving us the polymer.
Conclusion
Sometime around the year 1899, a German scientist while trying to decompose a compound called diazomethane found some sticky matter at the bottom of his flask. After great research, it was found that he had accidentally made a polymer of -CH2– molecules called polymethylene.
Later, in 1935 some British scientists while trying to react ethylene with benzaldehyde came across a waxy solid later to be known as polyethylene or commonly called plastic. Today, most of the cables are wrapped around with polymers of polyethylene for insulation.
Polyethylene is most commonly used by humans all over the world in various forms such as garbage bags, soda bottles, packaging, containers, etc. We’ve come a long way from stumbling across polymers to manufacturing them for specific industrial uses.
The next time while you work in a chemistry lab you might want to take a closer look at the residues that are formed after a reaction. It’s quite possible that you might discover a polymer too!
How well do you understand the article above!

