Table of Contents (click to expand)
Simply put, when wood comes in contact with fire, it undergoes thermal degradation, or pyrolysis. The pyrolysis of wood leads to the release of certain volatile gases and the formation of char, which eventually undergoes flaming and glowing reactions, respectively, to release the heat energy.
If you are the adventurous kind of soul who likes camping in the jungle, then you might have also relished sitting around a campfire, enjoying the sugary goodness of s’mores. If that sounds like you, then you should be grateful for the wood that had to burn for you to savor the treat.

We all know what happens when wood mingles with fire. It will first produce some smoke and then blaze with hues of orange and red. The stack of glowing embers finally fizzles out to form a heap of cooled grey ashes.
All this is what we observe at the surface level, but have you given any thought to what happens at the molecular level of the wood during its fiery end?
Composition Of Wood
The chemical composition of wood varies with the part, type and location of the tree the wood comes from. However, in a general sense, wood comprises two key chemical components: lignin (18-35%) and carbohydrates (65-75%). Carbohydrates such as cellulose (40-50%) and hemicellulose (25-35%) contribute to the majority of the wood composition.
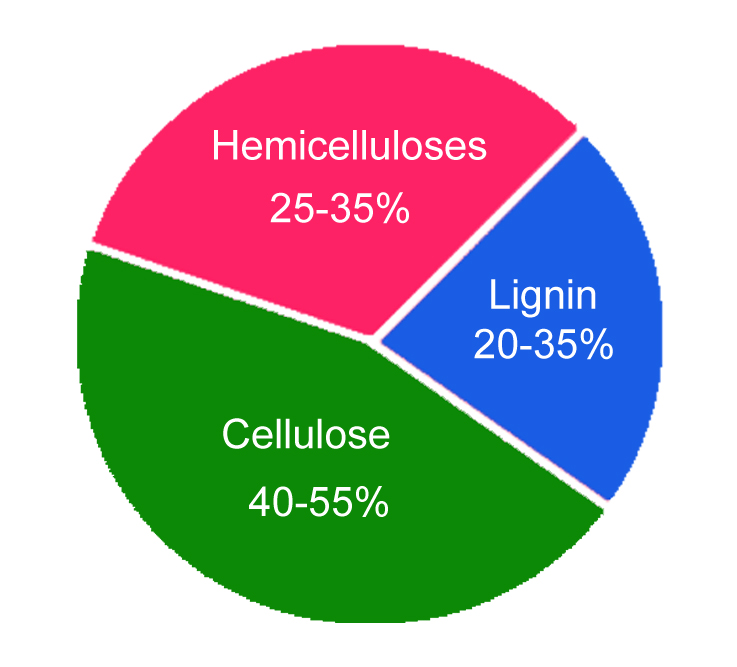
Apart from these complex molecules, the wood may also contain inorganic substances (ash) and organic extractives (phenolic compounds, fats, waxes, terpenes and terpenoids).
In a nutshell, the wood has an elementary composition of roughly 50% Carbon, 6% hydrogen, 44% oxygen and trace amounts of metal ions. Almost all species of wood have roughly the same elementary composition.
Also Read: Is It Possible To Melt Wood?
What Happens When Wood Burns?
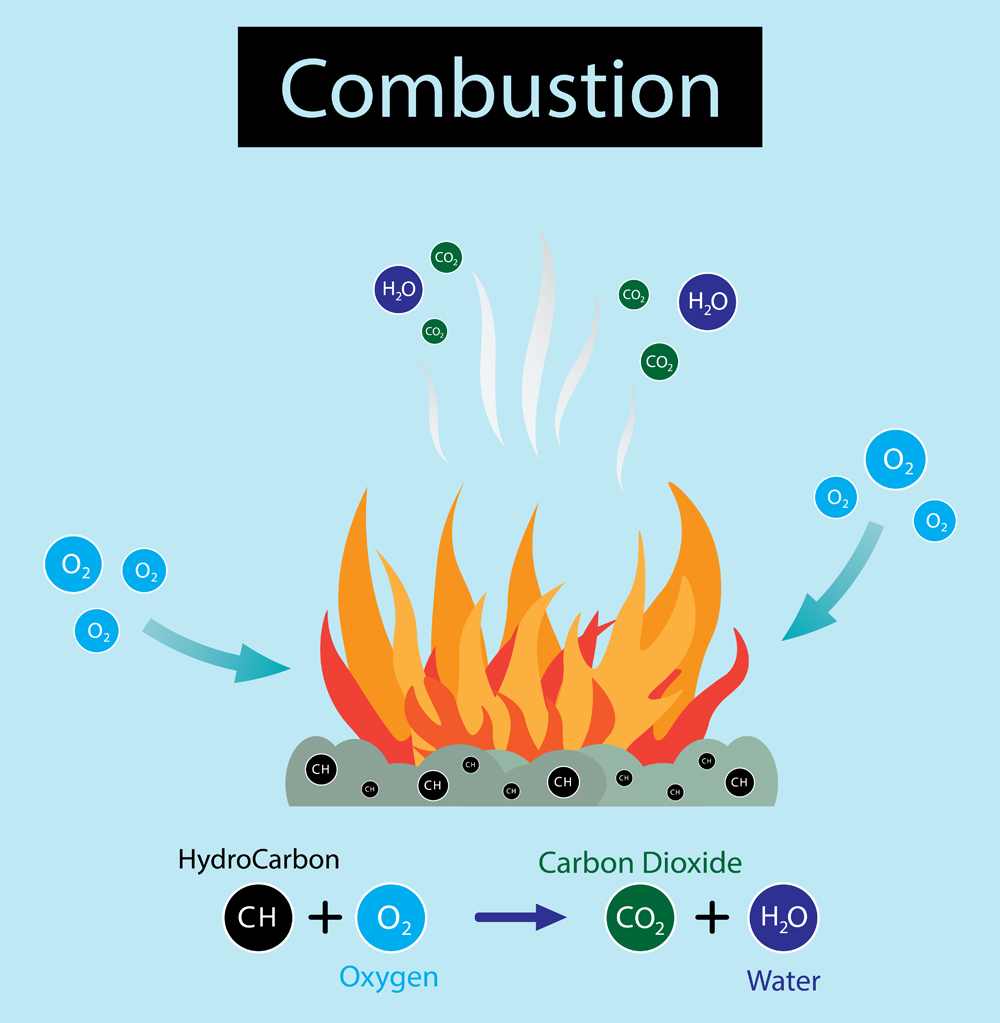
When you light wood on fire, it burns indirectly. Meaning, it undergoes a combustion reaction, rather than burning. That doesn’t make sense, does it? So what’s the difference between combustion and burning?

For combustion to take place, a fuel such as wood must be oxidized to release energy in the form of heat, but without flames. Whereas in burning (which is a type of combustion), the energy released is in the form of light (flame). Since most energy converts into light, the amount of heat produced is comparatively less than what is produced in combustion.
Simply put, when the wood comes in contact with fire, it undergoes thermal degradation or pyrolysis.
Also Read: Science Of Candles: How Do They Work?
Pyrolysis Of Wood
Pyrolysis plays a significant part in wood combustion. It is a process of thermal decomposition of organic matter under an inert atmosphere or in the absence of oxygen. Pyrolysis of wood leads to the release of some volatile gases and the formation of char, which eventually undergoes flaming and glowing reactions, respectively, to release the heat energy. Being a complex chemical reaction, the pyrolysis of wood occurs in three major stages.
Stage 1
To initiate the combustion of wood, a source of heat is brought in contact with wood in the presence of air. This causes a rise in the temperature of an area on the wood. As the temperature of the wood reaches 100°C, the water in the wood begins to boil and evaporate. As you may know, it is difficult to burn wood with a high moisture content. Therefore, dehydration of wood is essential for combustion to begin. At around 160°C, the dehydration is complete.
The hemicellulose in the wood starts to decompose between the temperature range of 200°C to 280°C. The decomposition causes the production of gases, such as carbon dioxide, carbon monoxide, acetic and formic acid.
The gases released during the first stage, do not catch fire until the moisture evaporates completely and the temperature is hot enough.
Stage 2
The second stage of wood combustion is a heat-producing stage. It begins when the temperature of the wood is above 280°C. During this stage, a large amount of energy, along with unburnt combustible gases like methane and methanol, water vapor and carbon dioxide is released. These gases are called secondary gases, and they comprise about 60% of the potential heat in the wood. The combustion of these gases is important for efficient wood combustion.
In this stage, the cellulose starts to decompose and reaches a peak at around 320°C. The decomposition of cellulose leads to char, tar and volatile products formation.
Stage 3
At temperatures beyond 320°C, the decomposition rate of lignin intensifies. At this point of wood combustion, all the gaseous products evaporate. The gas mixes with air to either cool off and form smoke, or catch fire to burn in flames. The only solid product of wood pyrolysis is the carbon chains of cellulose and lignin molecules left behind as charcoal. Around 20-30% of the weight of the wood converts into charcoal.

The charcoal bed continues to burn for a long time, but with low heat output. The glowing charcoal will radiate its energy away and cool off in the absence of oxygen. However, as we know, blowing air at the glowing charcoal causes it to burn brighter and spread the fire. Hence, the fire can be rekindled by supplying new oxygen to the charcoal bed and adding more wood.
A small percentage of wood that did not burn, known as ash, is left behind after all the combustible products are removed. Wood ash contains potassium carbonate, which acts as a good soil fertilizer.
Conclusion
Until now, you might have found the burning of wood to be a simple process where the fire burns every molecule that comes in its way, but I think it’s pretty evident that things are not as simple as they might appear!

When wood comes in contact with fire, it sets off a series of complex chemical reactions. The combustion of wood results in the release of carbon dioxide, water vapor and various gaseous products, as well as the formation of black solid residues like charcoal and ash.
How well do you understand the article above!

References (click to expand)
- Emmons, H. W., & Atreya, A. (1982, December). The science of wood combustion. Proceedings of the Indian Academy of Sciences Section C: Engineering Sciences. Springer Science and Business Media LLC.
- Theories of the combustion of wood and its control. Semantic Scholar
- (PDF) Modeling of pyrolysis in wood: A review - ResearchGate. ResearchGate
- Heating with Wood: Principles or Combustion. The University of Wisconsin–Madison
- The Chemical Composition of Wood - www.fpl.fs.fed.us
