Table of Contents (click to expand)
When you remove or add a neutron to the nucleus of an atom, the resulting substance is a new type of the same element and is called an isotope.
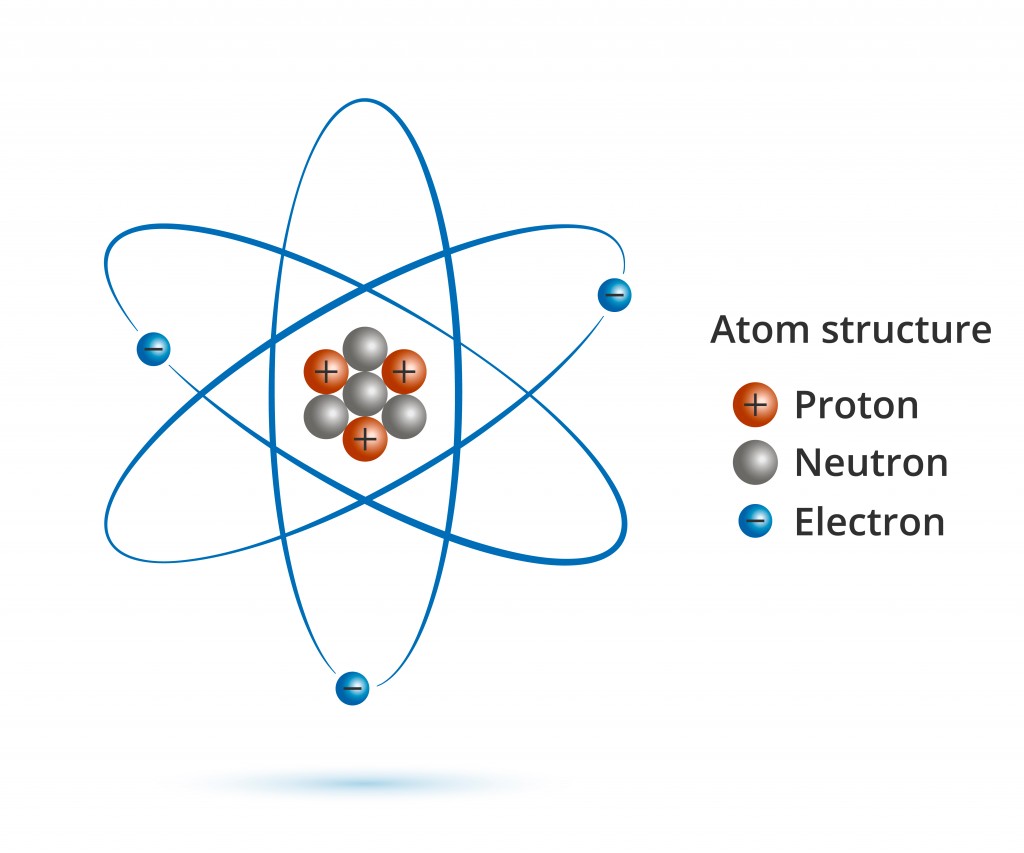
Everything you see around you is made up of matter, and all matter is made up of atoms. Can atoms be further broken down? Definitely. Atoms are made up of protons, neutrons, electrons and a bunch of different subatomic particles that most of us are unaware of.
Now, if everything consists of atoms, and atoms are itself made up of subatomic particles, would a change in the combination of subatomic particles result in a new, different kind of matter? It most certainly does.
If you were to remove an electron from an atom, you would be left with a positively charged ion, ready to bond with another oppositely charged ion. If a proton is taken out or added to an atom, a whole new element is formed (you would also need to add or remove the same number of neutrons to keep the nucleus stable). However, what happens when you remove a neutrally charged neutron from an atomic nucleus? A new element, perhaps?
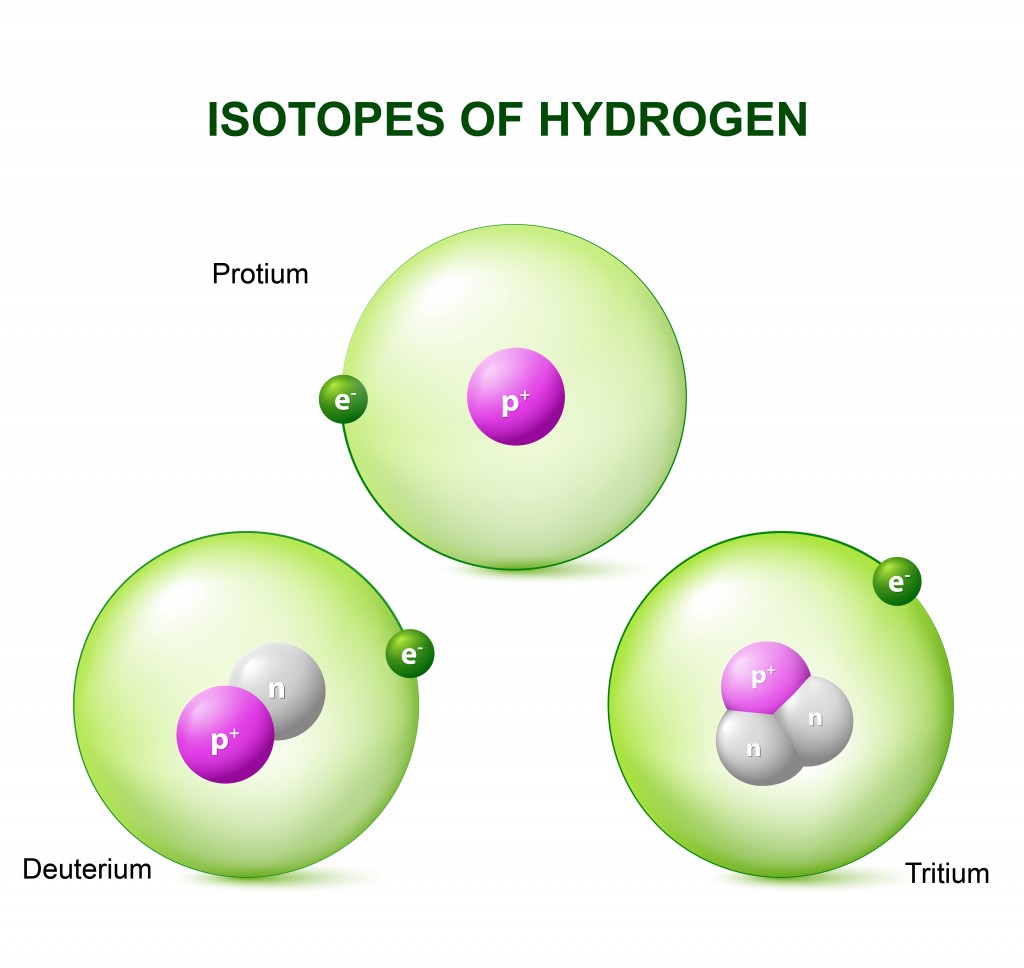
Isotopes
When you remove or add a neutron to the nucleus of an atom, you don’t technically get a new element, but you do have a new type of the same element in your hand. This new type is called an “isotope” of the element. Isotopes are formally defined as elements with the same number of protons, but a different number of neutrons in the nucleus. Another definition of isotopes defines them as atoms with the same atomic number (number of protons), but different atomic mass numbers (the sum of neutrons and protons of an atom). Isotopes of an element also have the same number of electrons.
Okay, let’s use the newly launched iPhones to understand the concept of isotopes in a more accessible way. Apple recently released three new devices in the Apple iPhone series, namely, iPhone 11, iPhone 11 Pro and iPhone 11 Pro Max. The Pro and Pro Max are nothing but a different variant or type of the iPhone 11. The two may be identified as a type of the iPhone 11 because they use the same A13 Bionic chip, or simply because they were released in the year 2019.
All the elements are identified solely based on their atomic number, i.e., the number of protons in the nucleus. So, similar to the iPhones, elements with the same number of protons (iPhones with an A13 chip), but a different number of neutrons (different number of cameras or battery size) are identified as different types of the same element, instead of an altogether new element.
Also Read: Why Do Elements Exist If They Have Such Short Half-lives?
History Of Isotopes
The existence of radioactive isotopes was first discerned by Frederick Soddy, a radiochemist, in the year 1913, while studying radioactive cascades (a series of radioactive decays). Soddy found 40 different element species (referred to as radioelements) between uranium and lead. However, the periodic table allowed for only 11 elements between the two. In an attempt to place these 40 elements in the periodic table, Frederick Soddy and Kazimierz Fajans came up with the radioactive displacement theory.
The theory proposed that alpha decay (radioactive decay in which an atom emits a helium nucleus, i.e., 2 protons and 2 neutrons) produces an element two places to the left of the parent element in the periodic table, whereas beta decay (emission of an electron or positron) results in an element one place to the right of the parent.
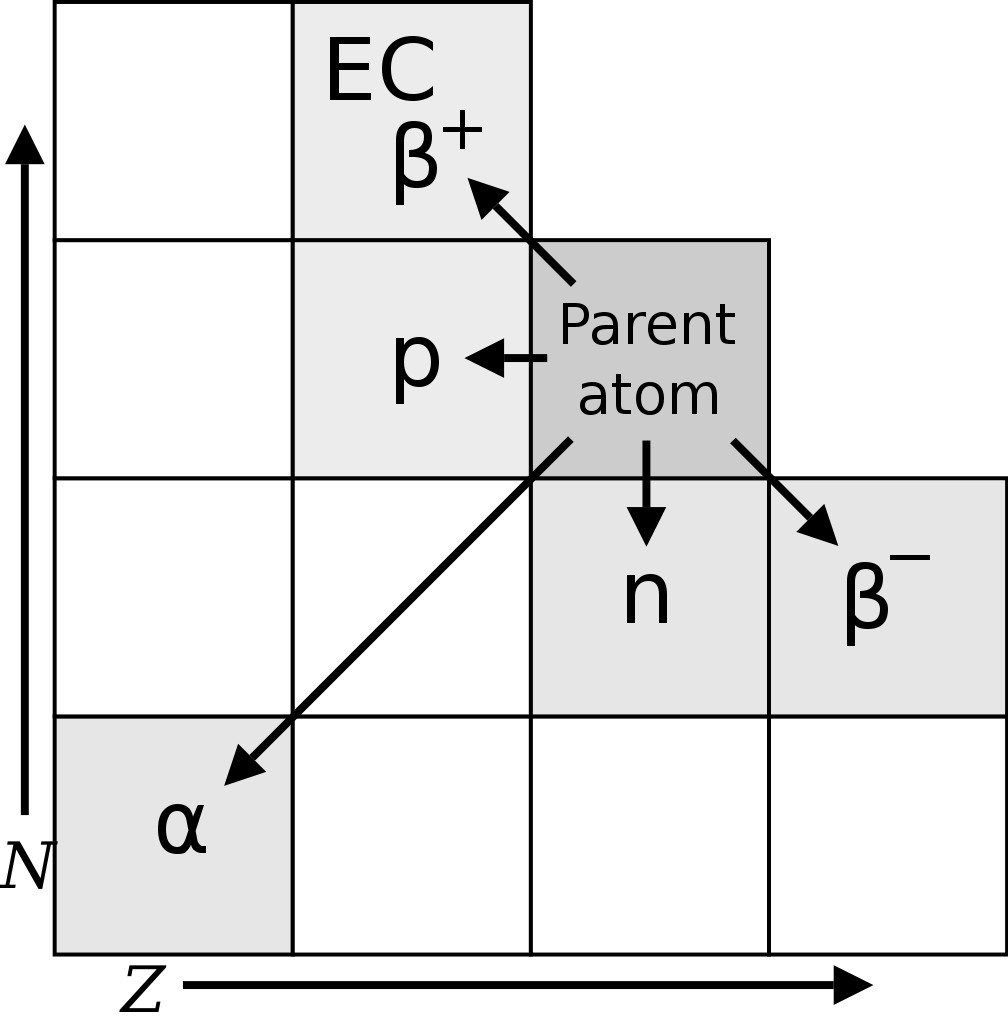
However, Soddy noticed that some radioelements had different atomic weights/masses, but retained the chemical properties of their parent element, and should, therefore, occupy the same place in the periodic table. Margaret Todd, a friend of Frederick Soddy, coined the term “isotopes” for these radioelements. The word isotope is Greek for “at the same place”. Soddy was awarded the Nobel prize for chemistry in 1921 for his contributions to the understanding of radioactive elements and the investigation and discovery of isotopes. J. J. Thomson found the first stable isotopes of an element in the year 1913.
Also Read: What Exactly Is Radioactivity ?
The Difference In Properties Between Isotopes
For simplicity’s sake, we can divide the properties of an element into two parts—chemical properties and physical properties. Chemical properties are the ability and tendency of an element, and thus its atoms, to undergo chemical reactions to form compounds or other elements. These properties are determined by the number of electrons present in the atom. Since isotopes of an element have a different number of neutrons, but the same number of electrons, they have similar chemical properties. However, the isotopes of hydrogen are an exception.
On the other hand, physical properties, such as the boiling point and melting point of an element, depend on its atomic mass number. A difference in the mass number lends different physical properties to an isotope from its parent. However, the nuclear properties of isotopes differ from one another and also form the basis of their classification.
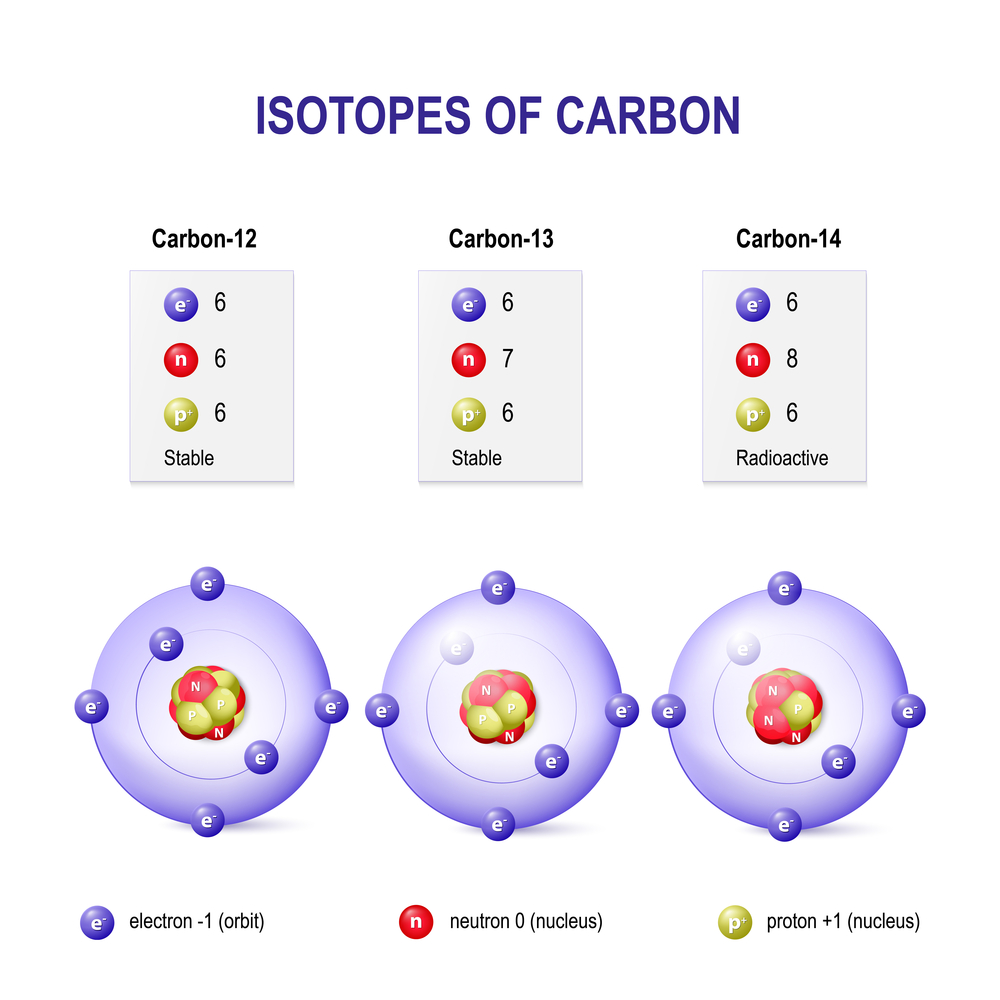
Isotopes are classified based on their stability, or tendency to decay. Out of the 339 naturally occurring isotopes, about 252 are considered stable. However, only 146 of them are actually stable, while the other 106 are theoretically susceptible to decay. Since no such observations of this have been made so far, we consider them observationally stable.
Also, out of the 339 naturally occurring isotopes, 286 are primordial, i.e., they have existed since the formation of the solar system. The grand total of isotopes confirmed so far, along with those created artificially, extends above 3,300. Over 2,400 of these have half-lives of less than 60 minutes. Every element has one or more radioactive isotope, while elements with atomic numbers greater than 83 only have radioactive isotopes.
Conclusion
This might be a fascinating subject, but removing neutrons from the nucleus of an atom is no walk in the park. Isotopes are primarily found on the used rods of nuclear reactors, as byproducts of nuclear reactions. They are artificially produced by bombarding stable atoms with alpha particles (helium nucleus) in a particle accelerator. Other methods include irradiating parent isotopes with neutrons in a nuclear reactor or using a cyclotron.
Radioactive isotopes find use in a variety of fields. The most promising is the discipline of nuclear medicine and the treatment of cancer. Industrial uses include homeland security, food irradiation, industrial radiography, geology, astronomy, etc. As we continue to discover more isotopes in the future, who knows what new uses we’ll find!
How well do you understand the article above!

