The Compton Effect is a quantum phenomenon that affirms the particle nature of radiation. This phenomenon is named after the physicist who discovered it.
The Compton Effect is a quantum phenomenon of scattering. American Physicist, Arthur Holly Compton, developed the theory. He studied the scattering effect of the X-rays that scattered off an electron. This backed the theory supporting the particle nature of radiation. He published this work in 1923, in The Physical Review. The article was titled “A Quantum Theory of the scattering X – rays by Light Elements”.

Note: ‘light elements’ here implies particles with very small masses, i.e., electrons.
Elastic Collision And The Compton Effect
When you are playing carom board or shuffleboard, you know exactly where to keep the striker to win a pawn… and then you shoot.
To shoot a pawn, it’s important for you to keep the striker at an appropriate angle, and hit it with an appropriate speed.
Of course, one must also aim!
As you observe the game, you will realize that the striker has kinetic energy that you supplied. After the striker has hit the pawn you are targeting, it would set that pawn into motion. There is a transfer of kinetic energy from the striker to the pawn.
This, in turn, changes the direction of the pawn.
The striker, having set the pawn in motion, would then undergo a reconciliation. There is also a loss of energy in the striker. This collision proceeds according to the conservation of momentum and energy principles. A collision where momentum and energy are both conserved is called an elastic collision.

Quantum Mechanics has been an answer to observations that classical physics couldn’t explain. The Compton Effect is one such phenomenon.
The Compton Effect And Particle Nature Of Radiation
The Compton effect was a breakthrough in the 20th century, as it confirmed the particle nature of radiation. Compton explained the particle nature of radiation through his scattering experiment.
The experiment found an increase in wavelength in the scattered radiation. He also detected that the scattered radiation had drifted from its initial angle. This form of elastic collision proved the particle nature of the radiation.
Now, let’s take a closer look at the experiment conducted by Mr. Compton!
Also Read: What Is Light? Matter Or Energy?
Experiment Demonstrating The Compton Effect
When an X-ray of wavelength  is incident on an electron, the scattered X-ray from the electron has a wavelength of
is incident on an electron, the scattered X-ray from the electron has a wavelength of  . The Compton Effect demonstrates an increase in the wavelength of the incident X-Rays.
. The Compton Effect demonstrates an increase in the wavelength of the incident X-Rays.
The Compton Effect scattered the X-rays off an electron, and this scattering complied with what we know of an elastic collision. Thus, it confirms the particle nature of radiation.
It concluded that the X-rays are made up of photons, and photons are discrete packets.
The wavelength of the scattered radiation is obtained through the Laws of Conservation of energy and momentum. The radiation after collision with the electron changed its direction of propagation. The electron loses some of its energy and experiences a recoil. The recoil provides velocity to the electron. This velocity is reflected as an increase in momentum.
The Compton Effect explains the relationship between the incident and the scattered wavelength.
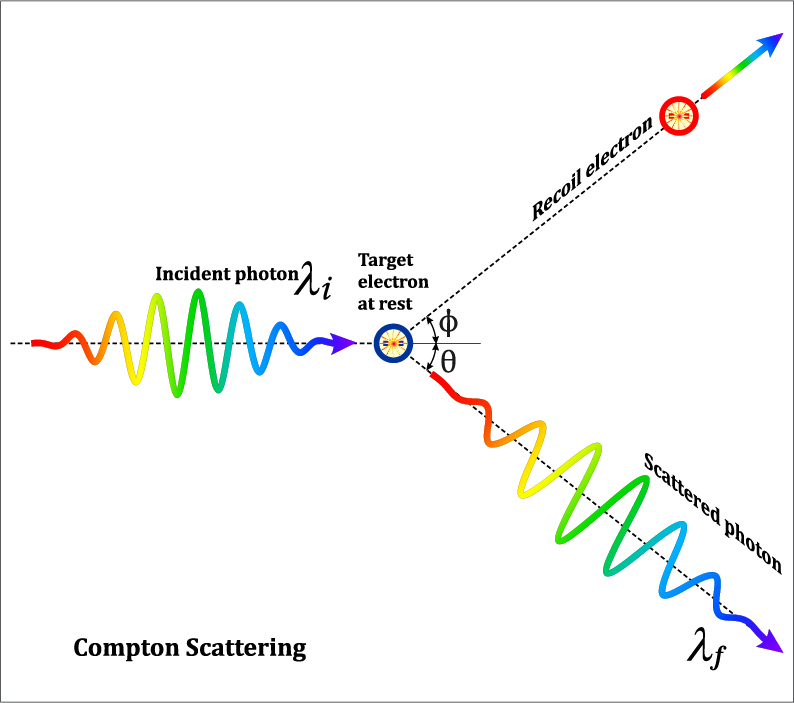
Compton Scattering (Photo Credit: Fouad A. Saad/Shutterstock)
Also Read: Wave-Particle Duality: Is An Electron A Particle Or A Wave?
The Mathematical Equation Of The Compton Effect
 (*)
(*)
 – the scattering angle, i.e., the angle between the incident ray and the scattered ray.
– the scattering angle, i.e., the angle between the incident ray and the scattered ray.

 – Compton wavelength and is equal to
– Compton wavelength and is equal to  m.
m.
where h is called Planck’s constant,  is mass of the electron and c is the speed of light in vacuum.
is mass of the electron and c is the speed of light in vacuum.
The above equation affirms that the scattering angle is independent of wavelengths of radiation, but is dependent on the increase in the wavelength.
Also Read: Do All Photons In The World Move At The Same Speed?
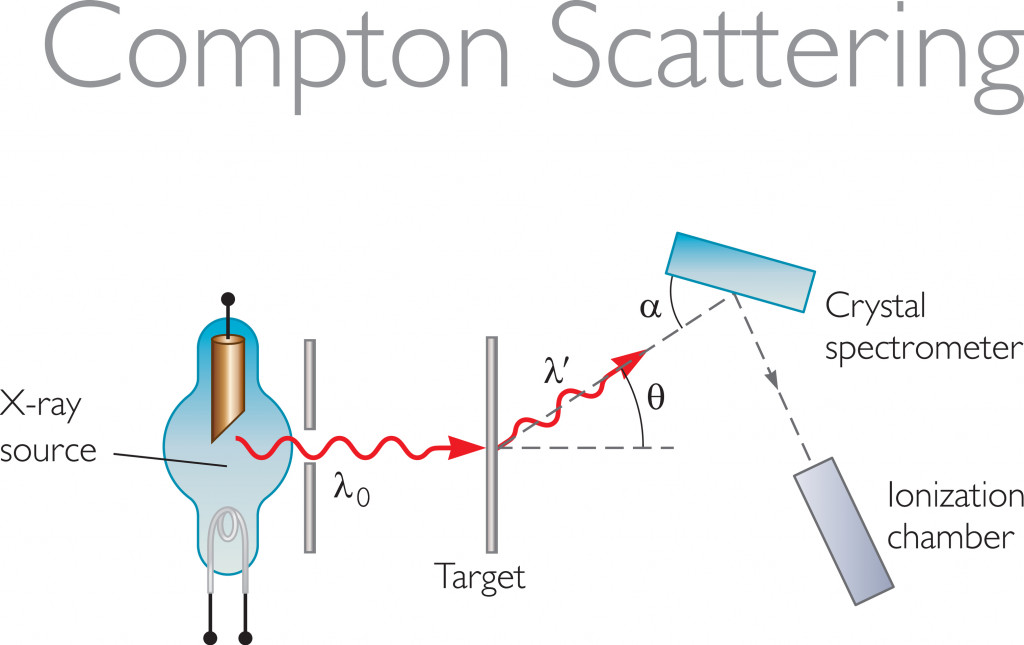
Comparing The Experimental Data With The Theoretical Values
Mr. Compton obtained experimental data by scattering electromagnetic rays on an electron. He allowed the X-ray radiation from a molybdenum target to scatter off graphite. The scattered radiation was measured at different angles, and these readings were plotted. The graph that was subsequently plotted was between the wavelength and the scattering angle.
The graph consisted of both wavelengths. The wavelength of the rays from the molybdenum target (the primary rays), and the wavelength of the scattered rays. The results were compared with the theoretical data that he developed.
The experimental and theoretical data matched!
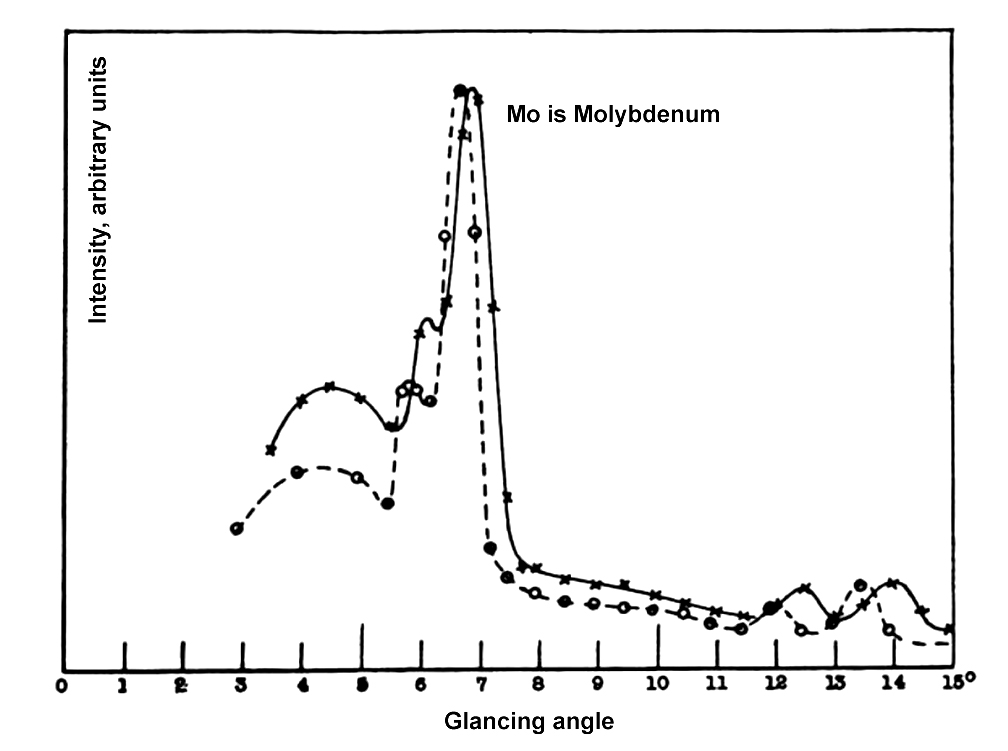
At a scattering angle of  , he observed the increase in wavelength to be
, he observed the increase in wavelength to be  m. The result from the theory was
m. The result from the theory was  m. He concluded that the observation was quite a satisfactory agreement. It emphasizes that the increase in wavelength is dependent on the scattering angle.
m. He concluded that the observation was quite a satisfactory agreement. It emphasizes that the increase in wavelength is dependent on the scattering angle.
The Compton Effect is one of the profound and most impactful theories that prove the particle nature of radiation.
Also Read: How Did We Learn That Carbon Has 6 Protons And Not 7?
Applications
The Compton Effect finds its application in radio-biology to treat cancer patients through radiation therapy.
Other applications of Compton Scattering include its use in the Compton Scatter Densitometer, which can be used to detect flaws or impurities in objects. Compton Scattering Imaging can measure the physical density of objects.
Today, numerous fields have emerged as applications of the Compton Effect. The 20th century, after Compton’s discovery, has been historical in the study and development of Quantum Mechanics. The Compton effect has been revolutionary in this area as well. It reiterates the idea of Particle-Wave Duality and opened new dimensions of human thinking in the early 1900s.
Also Read: What Is Blackbody Radiation?
How well do you understand the article above!

