Titration is a technique of determining the concentration of an unknown solution by using a solution of a known concentration. The known solution (titrate) is added in drops to the analyte (unknown solution) until the endpoint is reached.
Is lemonade your favorite drink? Or do you occasionally add it to your diet for weight loss? Whatever the reason may be, do you know what concentration of lemon juice you’re consuming in the form of lemonade? Aren’t you worried about causing damage to your own stomach if the concentration exceeds the limit?
First of all…Relax! You don’t have to worry about the concentration of lemon juice in your lemonade.
However, when it comes to certain drugs, food preservatives, the pH level of your fish tank, and in various industrial procedures, the concentration of different constituents in solutions does play a major role.
This is where titration comes into play! Titration determines the concentration of a solution by following certain strict procedures. This article explains the principle and methodology behind titration.

What Is The Principle Of Titration?
Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. By this process, the acid or base of a known concentration completely neutralizes the acid or base of the unknown concentration. The actual reaction that takes place during neutralization is between a hydrogen ion from acid and hydroxide ion from base that combine to form water. It is important to maintain a uniform pH throughout the process. Hence analyte and titrate should be of equal concentration. Involving strong titrate and a diluted analyte or vice versa will affect the physical of the analyte. The process is generally monitored by pH electrodes or indicators.
The solution of known concentration is the titrate and the solution whose concentration is to be determined is the analyte. The equivalence point of this process is obtained when the titrate completely neutralizes the base or acid in the analyte.
Also Read: Buffer Capacity: Definition And Method Of Calculation
How Is Titration Carried Out?
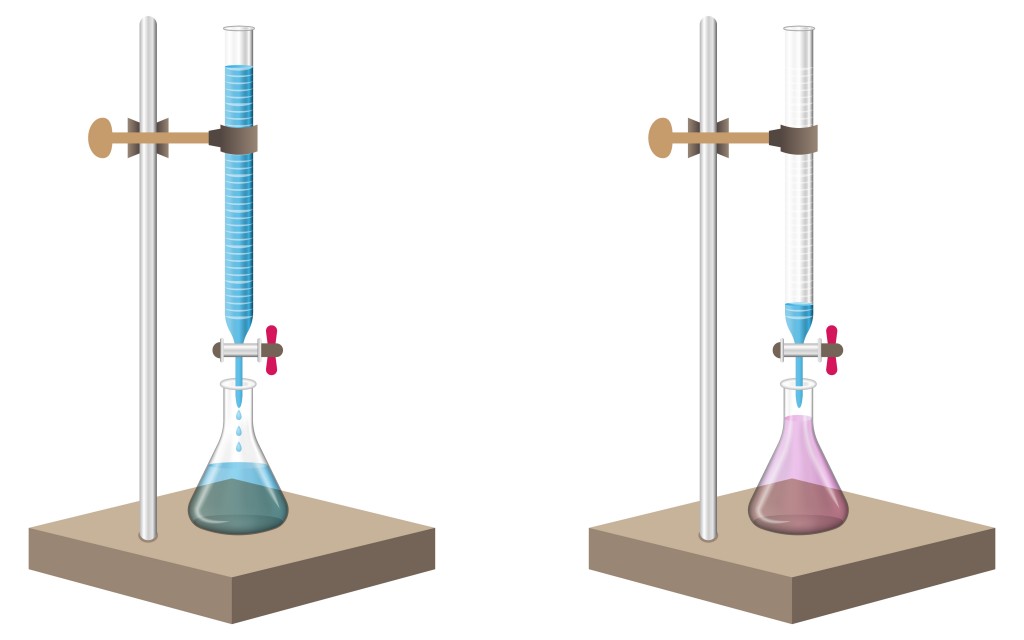
The analyte is measured and filled in a beaker. A few drops of an acid base indicator, such as methyl orange or phenolphthalein, is added to the analyte.
Phenolphthalein remains colorless in acid, but turns pink in the presence of a base. Similarly, methyl orange is a reddish-orange powder that turns to deep red in an acid and pales out to yellow in a base.
A standard solution is taken in a burette to start the process. The standard solution is allowed to drip slowly into the beaker containing the analyte. The process is carried out until the color of the analyte changes, indicating the arrival of an end point. This means that all of the base or acid in the analyte has been completely neutralized by the titrate.
The volume change of the standard solution at which the end point occurs is noted. This volume indicates the amount of titrate that has been used to neutralize the analyte. These values are then used in further calculations.
Also Read: Why Are pH Values Only In A Range Of 0-14?
Titration Formula: How Is Concentration Of The Analyte Calculated?
Like every other quantitative analysis, titration has a standard formula to determine the unknown concentration. The generally used formula is:
Volume of titrate * molarity of titrate = Volume of analyte * molarity of analyte
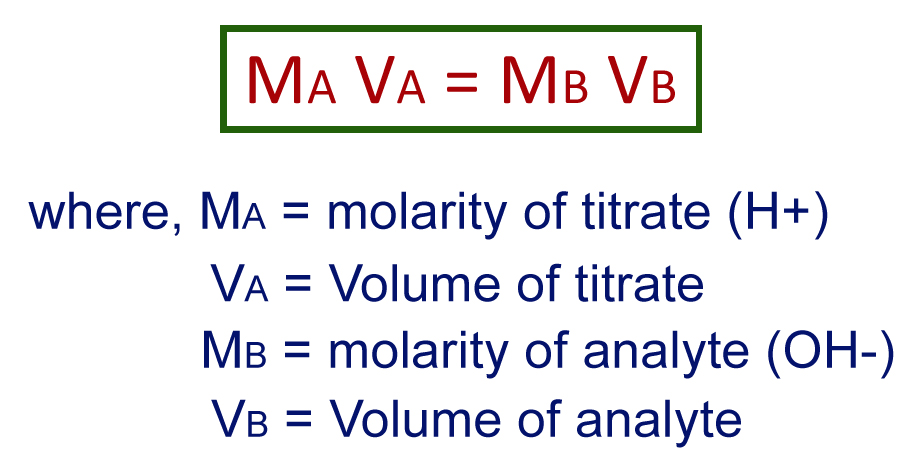
Let’s consider that 45.6ml of 1.25 M sodium hydroxide has been used to neutralize 20ml of hydrochloric acid. The concentration of HCl is then given by,
45.6 X 1.25 = 20.0 * molarity of analyte.
Solving the above equation will yield the concentration of the analyte.
Types Of Titration
Based on the type of reaction involved, titraion is classified into 4 different types.
- Acid-base titration: This is based on the neutralization between an acid or base and the analyte.

- Complexometric titration: This is widely used to determine the number of metal ions involved in the reaction. EDTA is used as an indicator. At the end of this titration, an undissociated complex is formed that marks the equivalence point. For example:
Ag+ + 2CN– → [Ag(CN)2]–
- Redox titration: In this type, the transfer of electrons takes place in the reacting ions of the solution. It is further classified into 3 types based on the type of reagent used.
Permanganate: MnO4–+ 8H + 5e → Mn2++ 4H2
Dichromate: K2Cr2O7+ 4H2SO4 → K2Cr2(SO4) + 4H2O + 3[O]
Iodimetric and iodometric: l2 + 2e → 2l–
- Precipitation titration: This reaction forms an insoluble precipitate when the two reacting substance are brought into contact.
AgNO3 + NaCl → AgCl + NaNO3
Titration Utility: Practical Applications And Examples
Titration is presumably applied in one way or another in our day-to-day activities. Various clinical tests, such as blood tests and urine tests, use titration to determine the concentration of chemicals of interest. It is also used in the food industry to determine the amount of certain chemicals in food.
Often, it is used to determine carbohydrate, fat and vitamin content. Titration is also widely applied in the medical field. Pregnancy tests, blood glucose level tests and other urinalysis applications use titration. It is also included as a part of academics for high school students to analyze their practical aptitude.
Sea water properties, such as the concentration of ammonia, nitrates and pH level are calculated and the environment is modified accordingly to maintain optimum conditions.
Concentration—commonly expressed in terms of molarity (number of moles of solute per liter in solution)—is of great importance in chemistry because it determines the rates of reaction and the conditions at equilibrium. Titration is the most prominent and widely used method to determine unknown concentration.
How well do you understand the article above!

