When a coffee stain dries, more evaporation occurs at the edges than at the center. This results in an outward rush of fluid, which leads to the deposition of particles along the edges.
Are you a coffee person? If you are, you probably have souvenirs of your “love for the drink” etched across your table, floor, book pages, and clothes… yes, I’m talking about one thing that every coffee lover dreads—Coffee Stains!
Now, take a closer look at one of them. You’ll notice that coffee stains don’t really end up as a dark brown blotch. Instead, they tend to leave a gentle tint of brown surrounded by a thin dark edge.
Have you ever wondered why the stain is distributed like that?
Coffee: Solution Or Suspension?
A basic cup of coffee is prepared by mixing ground coffee beans with hot water. A cup of this musky blend is the perfect potion to tackle a stressful day. Literally, coffee is a “solution” to all of life’s problems, but in the chemical world, is coffee also a ‘solution’?

Well, coffee consists of both dissolved and undissolved oils, and a fair amount of widely sized particles floating around. Hence, coffee is indeed a solution, but it is also fair to call it a suspension, or even a colloid.
Also Read: How Is Instant Coffee Manufactured?
The Coffee Ring Effect
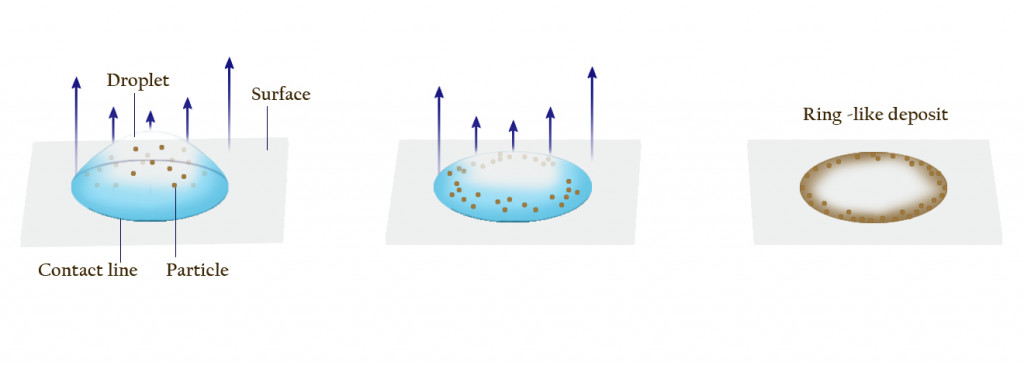
When you spill coffee, initially, all the suspended particles are distributed more or less uniformly throughout the splattered coffee drops
However, as the coffee dries, these tiny particles and coloring agents begin to accumulate along the edges, forming a ring around the stain. Scientists fancily call this phenomenon The Coffee Ring Effect.

But what forces these particles to the edge?
Also Read: Coanda Effect: Why Is It So Hard To Pour Liquid From Mugs?
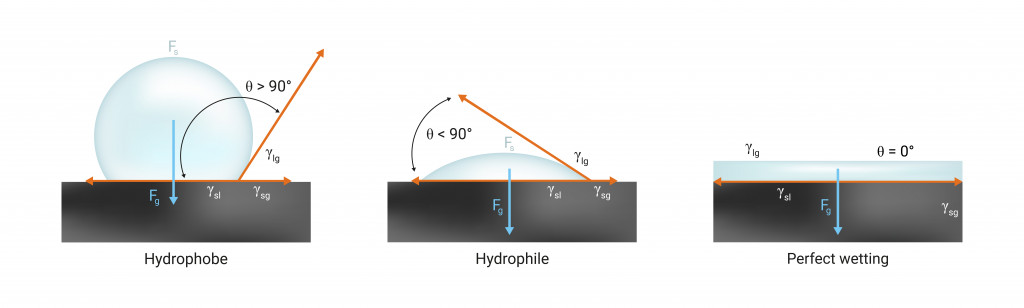
The Science Of A Coffee Blob: Cohesion, Adhesion, And Surface Tension
Molecules of fluids like coffee are very clingy. They always try to huddle up closely around other coffee molecules. This tendency of similar molecules to stick together is called cohesion.
Due to cohesion, the molecules on the surface of the liquid are pulled strongly towards the interior by the molecules in the bulk.
This strong tension experienced by the surface molecules is what we call surface tension. Surface tension makes the surface of the liquid contract and settle in a state where it has the least surface area possible. The shape that provides the best arrangement with the lowest surface area is a sphere.

Hence, cohesive forces always tend to keep a drop spherical!
However, we’ve never seen a perfectly spherical drop of coffee resting casually on a table. Why? Because the liquid molecules are not only attracted to each other, but also to the molecules of the surface. This kind of attraction between different kinds of molecules is called adhesion.

Contrary to cohesion, adhesion tends to flatten the liquid drop.
Depending on the strength of the cohesive and adhesive forces, and the nature of the surface, the blob can be closer to a sphere or a thin flat mess. Ideally, the drop ends up somewhere in between… as a spherical dome.
Once it reaches a stable state, the drop tries very hard to maintain it.
What Happens As The Drop Dries?
When a coffee drop falls on a surface, something interesting happens. The edge of the drop gets attached or “pinned” to the grooves on the surface. This ‘edge pinning’ is essential for the coffee ring effect to occur.
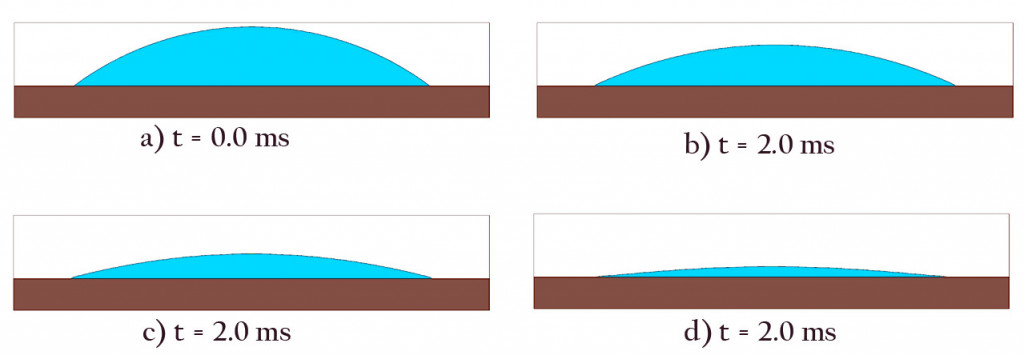
Once pinned, the edges of the drop don’t budge, and the liquid begins to dry via evaporation and a bit of absorption. However, the rate of evaporation is not even throughout the surface.
Studies show that the rate of evaporation is greater at the edges where the liquid comes in contact with both air and the solid plane.
Since the drop is thicker at the center and evaporation is greater at the edges, as drying progresses, the blob ends up looking like this:

However, as mentioned earlier, liquids are really picky about their shapes. The moment their flat dome shape is disturbed, they try to return to their stable formation. To achieve this, the fluids from the center rush out to the edges and refill the drying areas.
This process of replenishment continues throughout the drying process.
Thus, while drying, the outer border of the drop remains fixed, but it keeps getting flatter and flatter.
So… what about the coffee particles?
As the fluid keeps flowing outwards, the coffee particles also get washed up along the pinned rim. The spherical coffee particles then pack themselves tightly along the edges.
Thus, as the full amount of liquid evaporates, all that remains is a pile of dark-brown coffee particles that have accumulated on the border. This is what we observe as the darkened edge of a coffee stain.
Some Stains Are Patchy
Not all coffee stains end up as a perfectly dark ring. Some stains have a subtle border around them, while others will end up as a patchy mess.

As we said earlier, depending on cohesion and adhesion, the shape of the liquid drop varies, and in turn, the outward flow also varies. This affects the deposition of particles, so sometimes we end up with patches instead of rings.
Conclusion
The coffee ring effect is predominant in many other fluids aside from coffee. Eliminating or avoiding this effect is necessary in fields like coating, paint manufacturing, and printing, where the uniform deposition of color is indispensable.
Studies show that the presence of surfactants, like soap and ellipsoid-shaped particles, can mitigate this effect. Research is still ongoing in this field.
Now you know all about the science behind coffee stains, so the next time you accidentally spill some coffee on your science assignment, try diverting the teacher’s wrath by flexing your knowledge about the Coffee Ring Effect!
How well do you understand the article above!

References (click to expand)
- RD Deegan. Capillary flow as the cause of ring stains from dried liquid drops. The James Franck Institute of the University of Chicago
- Poulichet, V., Morel, M., Rudiuk, S., & Baigl, D. (2020, August). Liquid-liquid coffee-ring effect. Journal of Colloid and Interface Science. Elsevier BV.
- (2011) Suppression of the coffee-ring effect by shape-dependent .... The University of Pennsylvania
- (2012) Surfactant-Induced Marangoni Eddies Alter the Coffee-Rings .... The University of Pennsylvania
- (2013) Coffee Rings and Coffee Disks: Physics on the Edge. The University of Pennsylvania
