The molecules of alcohol have weak intermolecular force between them. This lack of strong H-bonding makes it difficult to freeze alcohol.
Have you ever wondered why a bottle of pure ethanol doesn’t freeze, even when you store it in the deep freezer? As you may know, alcohols are characteristically difficult to freeze into their solid states, as these organic molecules tend to stay in their liquid state. The solidification of different liquids depends on several factors.
But why do liquids solidify in the first place? And what conditions lead to it?
Since ethanol is the most common alcohol, this article will primarily focus on this type. The terms ethanol and alcohol will be used interchangeably in this article.
Why Do Liquids Freeze?
Freezing can be simply defined as a compound transforming from its liquid to its solid state, but what causes liquids to undergo this process?
Every compound has some internal energy that is dependent on the temperature around it. This internal energy controls the state in which the substance exists. When the temperature around the compound reduces, the internal energy available to the compound reduces as well. This leads to an increase in the intermolecular forces.

When heat is removed, the molecules of the compound come closer together. This is why many liquids begin to solidify at a low temperature. However, different liquids have different freezing points.
The freezing point of a liquid is the temperature at which that liquid transitions into a solid state. Water has a freezing point of 0° Centigrade, but ethanol has a freezing point of -114° Centigrade. This means that to freeze alcohol, you have to drop its temperature below -100° C!
The huge difference in the freezing point of the two liquids is due to the huge difference between their intermolecular forces. The molecules of water are bound more tightly to each other than the molecules of ethanol.
Also Read: Why Does Water Expand When It Freezes?
Intermolecular Forces And Hydrogen Bonds
Intermolecular forces help bind the molecules to each other so they do not separate. Gases have the lowest amount of intermolecular force, whereas solids have the highest. The explanation for this is rather simple. The intermolecular forces between molecules in the liquid state are less than those in a solid state, but more than in gases.
These forces are dependent on the distance of the molecules from each other. The distance is the highest in gases, and lowest in solids. Intermolecular forces are also dependent on the nature of the molecules. Hence, some molecules inherently have greater intermolecular forces.
The most important bond displaying strong intermolecular force is something called H-bonding. The hydrogen atom of one water molecule forms an intermolecular H-bond with the oxygen atom of another molecule. Sometimes, hydrogen atoms make stronger bonds, depending on the atom to which they are connected.
Also Read: What Are The Bubbles Made Of When Water Boils?
Hydrogen Bonds In Water And Ethanol: A Comparison
For example, let’s take a look at the structure of water:
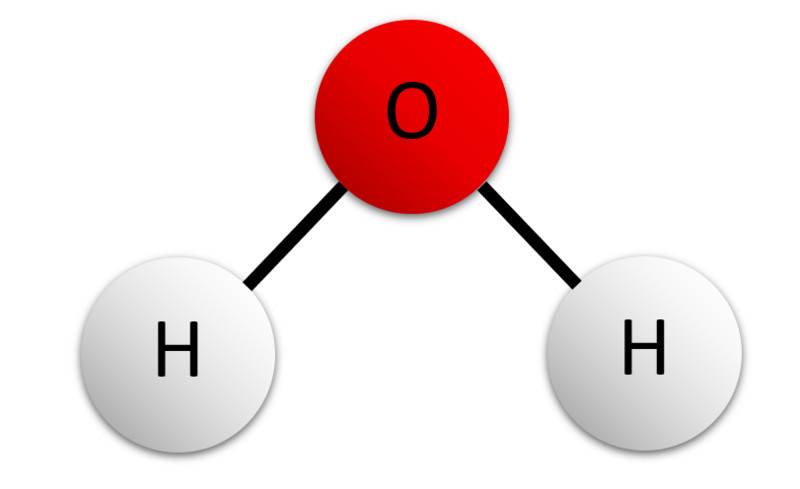
Here, you can see one oxygen atom bound to two hydrogen atoms. Now, these two hydrogen atoms must bind to the oxygen of another molecule, which gives rise to a structure like this:
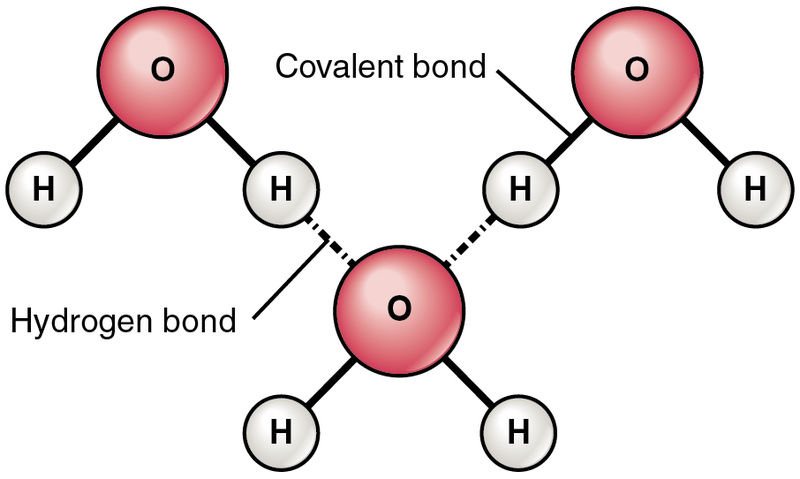
Now, let’s see what an ethanol molecule looks like:
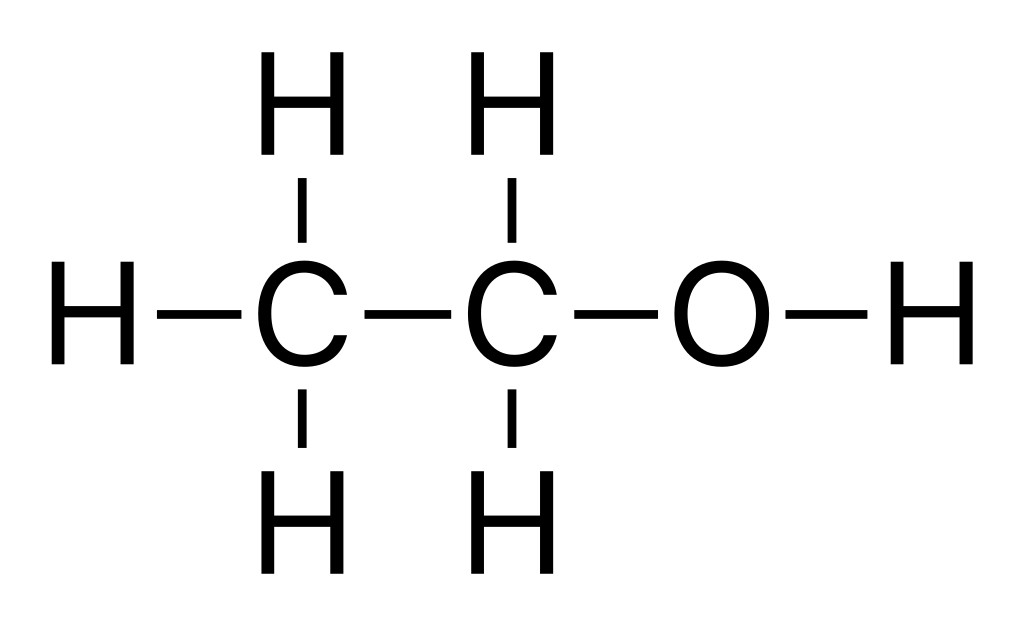
An ethanol molecule has two carbon atoms attached to each other. Each carbon atom can make three more bonds. The first carbon has three hydrogens, while the second has two hydrogens and one alcohol (-OH) group attached. It is this -OH group that forms the hydrogen bonds between alcohol molecules.
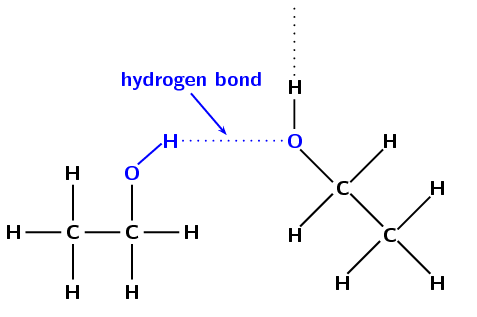
However, since the oxygen is connected to a carbon, the polarity of the oxygen goes down. Polarity here is the amount of negative or positive charge that an atom possesses in a neutral molecule.
The more polar the atoms in a molecule, the more polar the molecule becomes. Here, water is much more polar than ethanol, which is why the H-bonds in water are so much stronger.
Also Read: Why Are Sugary Solutions Sticky?
How Does This Affect Freezing?
Coming back to our initial question: why is ethanol hard to freeze? We know that alcohols have very little polarity, or are non-polar. The intermolecular forces between individual ethanol molecules are very low.
Since those intermolecular forces are low, the molecules tend to stay away from each other. Until the molecules come together, the compound cannot exist in a solid state.
The ethanol molecules do not come together on their own. Extremely low temperatures are needed to solidify the compound. Simply put, the lack of attraction between the molecules makes it difficult for alcohol to freeze.
Conclusion
As compared to liquids like water, it is much more difficult to freeze alcohol. Even in the -15 °C of a common home freezer, a bottle of pure ethanol will refuse to solidify.
This is because ethanol is an organic, non-polar compound and cannot make strong hydrogen bonds. This affects the intermolecular forces and makes them weak. Since the H-bonds and intermolecular forces are weak, the ethanol molecules refuse to cooperate and join together.
This leads to the compound having higher energy, so it needs a much lower temperature to solidify. This is why it is so difficult to freeze alcohol!
Also Read: Why Does Ice Float On Water?
How well do you understand the article above!

