Carbon dioxide is an IR active molecules that absorbs long infrared radiations emitted by the Earth’s surface.
It was the summer of 1856 and Eunice Foot was trying to identify the factors that influenced the heat from the Sun’s rays. Her experiments led to the conclusion that a closed environment rich in carbonic acid gas (now known as carbon dioxide) heated up much faster in sunlight than one with regular air. It also cooled down much slower when removed from direct sunlight.
In her paper, which she wasn’t even allowed to present because she was a woman, she wrote: “An atmosphere of that gas would give our Earth a high temperature, and if some suppose, at one period of its history the air had mixed with a larger proportion than the present…”. This observation didn’t get much attention back then, but is staring back at us every day. That’s because we are apparently living in the not-so-pleasant future Eunice had imagined.
Unless you’ve been living under a rock for over a decade, you know that carbon dioxide is a greenhouse gas responsible for global warming (although technically, “we” are responsible). But what qualifies it to be a greenhouse gas, while the other major components of air are not? Let’s get to know how the gas that makes your soda pop also makes glaciers plop!

A Brief History Of Greenhouse Gases
Every day our planet receives a quintillion Joules of energy from our favorite fiery medallion in the sky. Life-giving sunlight is a cocktail of Ultraviolet, Visible and Infrared rays.
Before all the rays reach the Earth’s surface, our atmosphere, a 55 quadrillion ton gas blanket floating above our head, filters out ~99% of the UV rays, with the help of the ozone layer (not 100%, so don’t forget sunscreen). It then lets in the visible rays that light up our world. Last but not the least, the infrared rays make the earth a warm and cozy pocket of life in the vast cold void.
The infrared rays that hit the surface of the earth are absorbed by different objects and radiated back out in the form of heat. The reflected heat tries to move away from the heated surface to the colder regions in the sky, which is when they face the gatekeepers of heat—the greenhouse gases.
Certain gases like carbon dioxide, water vapor, oxides of nitrogen, methane, and chlorofluorocarbon prevent the heat from completely escaping into space. If not for them, our planet would be a frozen ball of ice with an average temperature of 18 degrees below freezing!

Also Read: What If We Did Not Have Greenhouse Gases?
What Makes Carbon Dioxide A Greenhouse Gas?
In this context, when we say infrared or IR radiations, we mean the IR rays reflected by Earth’s surface, not the ones that enter with sunlight.
The major components of air, such as nitrogen and oxygen, are transparent to IR radiation, meaning that they do not interact with those rays. However, carbon dioxide or CO2 gas is IR active, meaning that it undergoes some chemical interaction with the IR radiation that stops it from leaving the planet (not all of it though). So what happens when these molecules interfere with the path of IR rays? For that, we need to zoom into the individual gas molecules.
Gas molecules are in a constant state of vibration, even under normal temperature and pressure conditions. These movements become more intense when hit by an external energy source. Now, imagine a CO2 molecule where carbon and oxygen atoms are ping-pong balls and the bonds connecting them are springs. Under normal circumstances, these bonds are bending and stretching at a particular frequency and hanging out in the atmosphere.
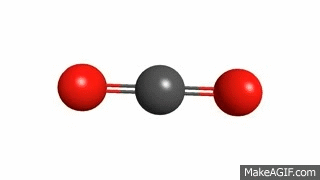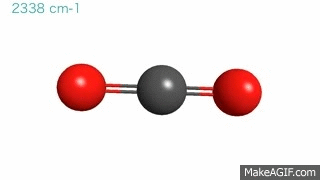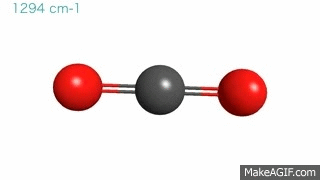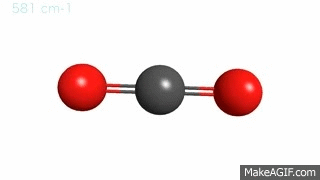
Then…. BAM! A photon of IR radiation hits the gas molecule, which soaks up the photon, gets excited, and starts vibrating at a faster speed. However, the gas molecule cannot keep up this faster motion for long and must relax back to its original state. It relaxes by emitting the energy back into the air or by transferring it to a nearby CO2 molecule.
The same phenomenon takes place over and over for trillions of CO2 molecules. The continuous absorption, excitation, and re-emission of energy is what traps the heat inside.
Why Aren’t Nitrogen And Oxygen Greenhouse Gases?
In every molecule, there are positive and negative charges due to the nucleus and electron clouds. When heteroatomic molecules like carbon dioxide, methane, or nitrogen dioxide vibrate, there is a shift in their charge distribution. Sometimes they are evenly distributed, and sometimes not. The unequal distribution of charges between bonds creates an electric field that makes them sensitive to electromagnetic radiations, like IR.
However, in the case of diatomic gases like N2 and O2, even when the bonds are stretching, there is no change in the electric field. Thus, the electromagnetic radiations pass them unhindered. Also, molecules are very choosy when it comes to which frequency of radiation they interact with. CO2 easily absorbs lower energy long wave IR radiation, but N2 and O2 only absorb higher energy radiations like Gamma or X-rays.
Also Read: Why Has Life Evolved To Depend On Oxygen Instead Of Nitrogen?
Is CO2 The Most Dangerous Greenhouse Gas?
A single molecule of chlorofluorocarbon can create a footprint equivalent to 10,000 molecules of CO2, methane can absorb 30 times more heat, and water vapor is the strongest amongst all the greenhouse gas present in the air.
Even though these gases are far more powerful as greenhouse gases than CO2, their concentrations aren’t affected drastically by human activity. The same cannot be said for CO2, as it is a major byproduct of many manmade activities. There has been a 90% increase in CO2 emissions since 1970, so while it is not inherently the most dangerous greenhouse gas, it has become the focal point of concern due to its excessive and largely unregulated release into the atmosphere.
Also Read: Impact Of Increasing CO2 Emissions On Environment
Conclusion
Carbon dioxide is a very important factor in maintaining our status as the Goldilocks planet. It has kept our waters liquid and our home habitable, but the summers keep getting hotter with every passing year because of the imbalance we have introduced via excessive emissions. Fortunately for us, nature has provided us with colossal carbon sinks in the form of soil, forests, and oceans. The least we can do is preserve and restore them and let them do their job!
How well do you understand the article above!

References (click to expand)
- Earth Temperature without GHGs - Energy Education. energyeducation.ca
- Is carbon dioxide a major contributor to global warming?. The University of Wisconsin–Madison
- Greenhouse Gases Absorb Infrared Radiation. Elmhurst University
- The Greenhouse Effect - CES/FAU. Florida Atlantic University
- Chapter 4. Greenhouse Gases. The University of Chicago
