Table of Contents (click to expand)
Manganese oxide is the inorganic compound with the chemical formula MnO. It is a blackish-brown solid that occurs naturally as the mineral pyrolusite. Manganese oxide is used as a catalyst in the production of allyl alcohol, paints, colored glass, and ceramics. It is also a component of fertilizers and food additives.
The term ‘manganese oxide’ is used to refer to any of the various kinds of manganese oxides that exist in nature. In addition to that, ‘manganese oxide’ may also refer to some manganese minerals, including hausmannite, birnessite, manganosite, manganite etc.
Manganese
Manganese (symbol ‘Mn’) is the 10th most abundant of all the elements found naturally on Earth’s crust. It is second only to iron as the most common naturally-occurring heavy metal on the planet. It is found in huge quantities in melts and deposits (like pegmatites).
Manganese is depleted from metamorphic and igneous rocks by interaction with groundwater and surface water. Another interesting quality of manganese is that it is easily oxidized, which makes it produce more than thirty known manganese oxide or hydroxide minerals.
Also Read: How Is Aluminum Made?
What Is Manganese Oxide?
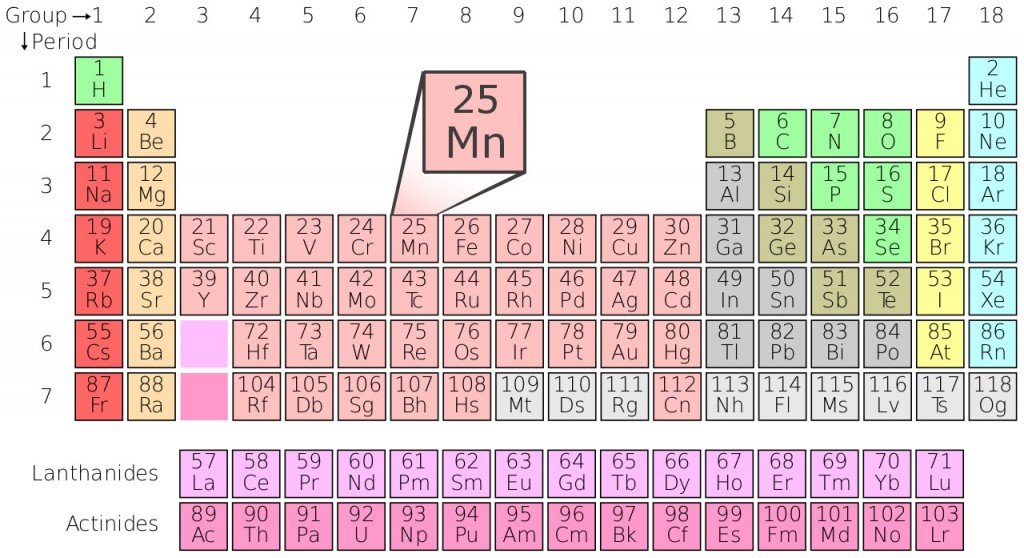
The term ‘manganese oxide’ can be used to refer to any of the manganese oxides and hydroxides, which include, Manganese (II) oxide (also called ‘Ferrite Grade’), Manganese (II,III) oxide, Manganese (III) oxide etc.
There are also several minerals that are casually referred to as manganese oxides, such as, manganosite, birnessite and manganite, just to name a few.
It’s pretty evident that there’s more than one ‘manganese oxide’ out there, but those that are most frequently associated with that name are manganese (II) oxide (MnO2) and manganese dioxide (MnO).
Also Read: What Is Oxidation?
Manganese (II) Oxide
Manganese (II) oxide is actually an inorganic compound, and has the chemical formula MnO. Like many other monoxides, it has a rock salt structure, which means that both the cations and anions are octahedrally coordinated. This picture below will help you visualize the structure of manganese oxide.
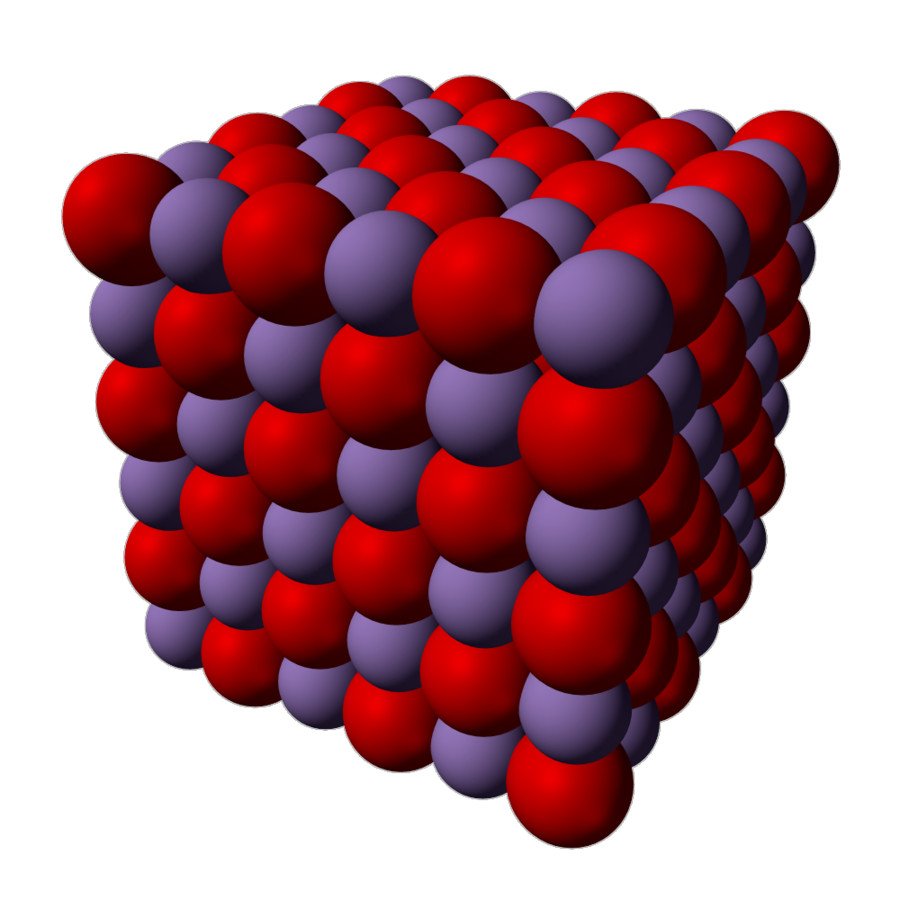
During chemical reactions, manganese (II) oxides behave like a typical ionic oxide; as such, when it interacts with an acid, it converts into the corresponding manganese salt and water.
Uses
Manganese oxide is a well-known component of fertilizers and food additives. Due to its effectiveness in the fertilizer industry alone, its annual consumption lies in the range of thousands of tons. Furthermore, manganese (II) oxide is used as a catalyst in the production of allyl alcohol, paints, colored glass, ceramics etc.
Manganese (IV) Oxide Or Manganese Dioxide
Manganese (IV) oxide is an inorganic compound. Its chemical formula is MnO2 and it occurs naturally as the mineral pyrolusite, which happens to be a component of manganese nodules.

Most people associate manganese oxide with the black innards of a dry-cell battery. However, it’s interesting to note that the oxide occurs in a wide variety of other places and geological settings: it’s found in soils and sediments, where it exists as fine-grained aggregates, as well as freshwater and marine nodules and concretions.
However, the most abundant deposition of manganese oxides occur in the oceans as nodules. Manganese nodules are found at almost all depths in every ocean and sea of the world. Just to give you some estimate of the ubiquity of Mn modules, it’s been estimated that manganese nodules cover around 10-30% of the deep Pacific floor!
Uses
The main and most common application of manganese oxide is seen in dry-cell batteries (zinc-carbon batteries or d Leclanché cell). The batteries account for a significant part of the total annual consumption of manganese oxide. In recent years, however, alkaline batteries, which also use manganese oxide, have begun to dominate the market.
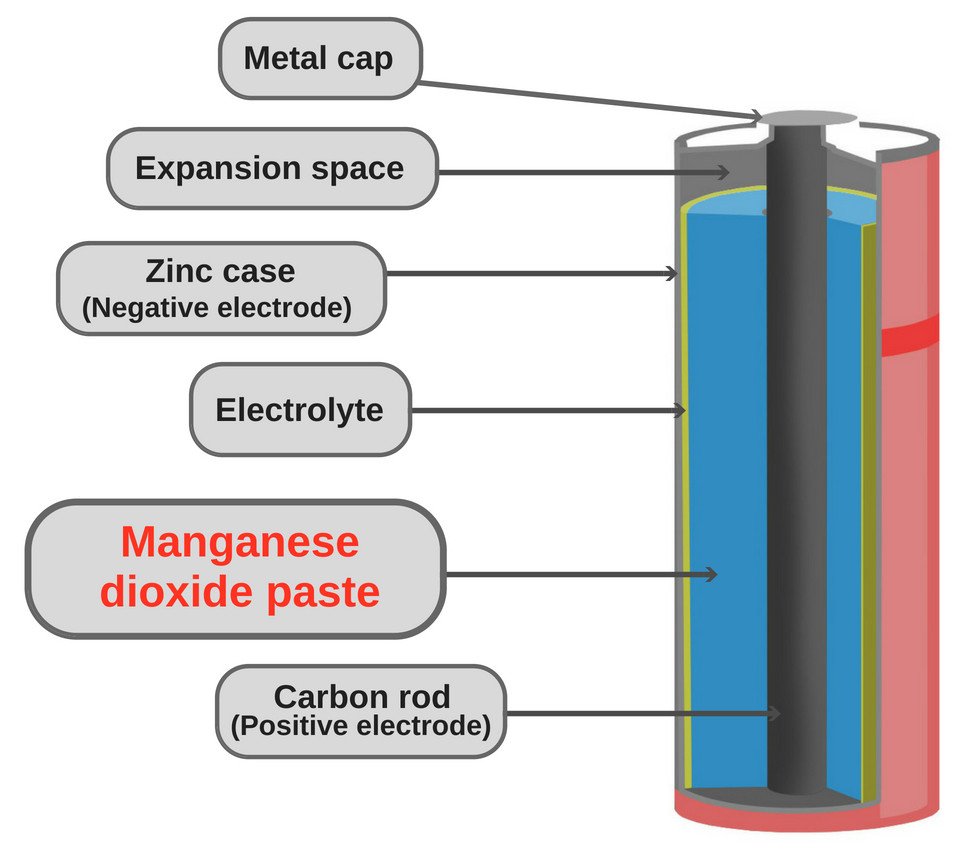
Additionally, it’s used as an additive for livestock feed, colorant for bricks, plant fertilizers etc.
An important thing to note about manganese oxide is that when it comes in contact with air and becomes contaminated, its particles can cause manganese poisoning or manganism, which can lead to a host of psychiatric and motor function disturbances.
How well do you understand the article above!

References (click to expand)
- Z Yin. Ferroportin is a manganese-responsive protein that decreases .... The University of North Carolina at Greensboro
- Fored, C. M. (2006, February 1). Parkinson's disease and other basal ganglia or movement disorders in a large nationwide cohort of Swedish welders. Occupational and Environmental Medicine. BMJ.
- (2002) Manganese oxide cathodes for rechargeable batteries. The University of Texas at Austin
- Manganese Oxide | Suib Group Website - wp.suibgroup.uconn.edu:80
