Table of Contents (click to expand)
- The Free Energy
- The Parameters In Thermodynamics
- Application Of Thermodynamics
- Work And Heat
- Mathematical Expression Of The First Law
- Mathematical Relation Between Enthalpy And Internal Energy
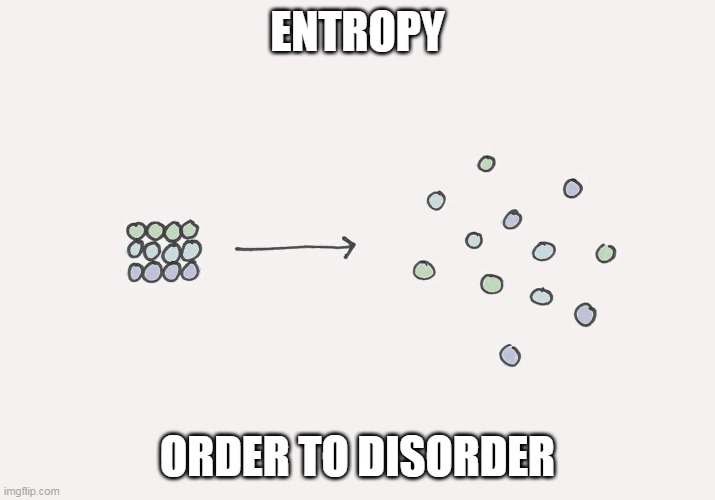
- Entropy
- Gibbs Free Energy
- The Relation Between Gibbs Free Energy And Work Done
- Mathematical Form For Gibbs Free Energy
- Categories Of Chemical Reactions Based On Gibbs Free Energy
“The free energy” signifies the maximum energy that is available, or free, to do useful work. Gibbs free energy is named after physicist Josiah Willard Gibbs, who proposed this method.“
Oftentimes, after you’ve cleaned your room, you observe that it gets cluttered again within a few days. Why should the room always need to be tidied up?
Next time your mom lectures you about this, perhaps you can tell her about entropy and Gibbs free energy!
The entropy of cluttered objects is more than the entropy of ordered objects. The spontaneity of any process or reaction is always moving towards higher entropy, which is your room has the tendency to get messed up so rapidly!
Nevertheless, you have a new excuse for your unkempt mess.
The Free Energy
Josiah Williard Gibbs, an American mathematician and physicist, developed the concept of free energy. The method of free energy proposed by him is now widely known as Gibbs free energy. In the year 1873, his publication “Graphical Methods in the Thermodynamics of the Fluids” introduced a simpler form of representation to understand various systems.
Back then, he referred to this free energy as energy/available energy (ε), which eventually gained popularity as Gibbs free energy.

Also Read: What Is Maxwell’s Demon?
The Parameters In Thermodynamics
In thermodynamics, we can understand the behavior of a system without getting into details of the composition or atomic/molecular structure of the matter itself. We understand the system with parameters/functions like Volume, Pressure, Temperature, Enthalpy, Entropy, Work Done and Gibbs Free energy. Thus, thermodynamics relates the properties of the given system to its behavior in terms of physical and chemical processes.
Also, it is concerned with equilibrium states of the system. In the equilibrium state, the macroscopic properties of the system, such as temperature, density and chemical composition, are well-defined and time-invariant. Thermodynamics is concerned only about the transition of the system from one state to another, rather than the path the system takes to reach that final state. Furthermore, the parameters—pressure, volume and temperature—help us understand the system’s behavior. These parameters are path-independent and referred to as state functions.

Also Read: What Is The Third Law Of Thermodynamics?
Application Of Thermodynamics
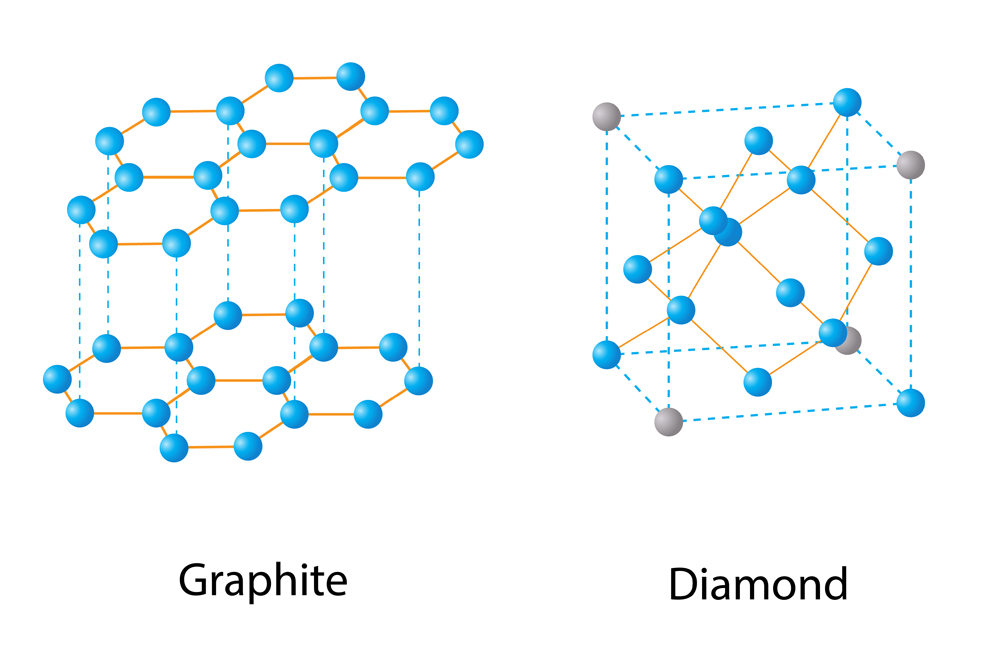
If the application of thermodynamics shows that any given system or chemical reaction is impossible to achieve, then it can never be accomplished in the laboratory, or rather, it has not been accomplished in the laboratory yet. A notable example for the application of thermodynamics has been in converting graphite into diamond. Thermodynamics showed the possibility for such a conversion under certain extreme conditions of temperature and pressure. However, scientists only achieved this feat after a long series of failures. Thus, thermodynamics can be an assurance for scientists to never give up!
When trying to infer about a system under study, through thermodynamics, it is important to obtain expressions for work and heat.
Also Read: What Is The First Law Of Thermodynamics?
Work And Heat
Work is described as the energy transferred by virtue of a mechanical link between systems and their surroundings. It is path-dependent and not a state function. It is useful to study hypothetical systems, but in the real world, there is yet another way that energy is transferred between the system and the surroundings. This energy transfer is by virtue of the temperature difference between the system and the surroundings, referred to as heat. Heat is transferred from the medium of higher temperature to the medium of lower temperature.

There are three laws in Thermodynamics. The first law is the principle of conservation of energy.”Energy can neither be created nor destroyed, but can be converted from one form to another.”
Mathematical Expression Of The First Law
Heat absorbed = increase in internal energy + Work done by the system

Work done by the system is negative and is expressed as ‘–W’, while work done on the system is positive and is expressed as ‘+W’.
The internal energy is the sum of the kinetic and potential energy of all the particles in the system.
Sometimes, when one is dealing with systems at constant pressure conditions, there is a practice to use the enthalpy of a system in place of the internal energy of the system.
Mathematical Relation Between Enthalpy And Internal Energy
The total energy stored in a system (enthalpy) = internal energy + product of pressure and volume
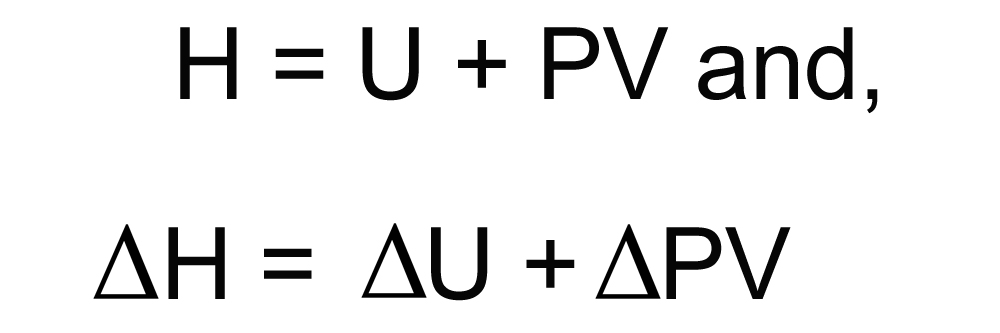
when Pressure (P) is constant.
This is the second law of thermodynamics and takes into consideration the concepts of spontaneity or feasibility of certain systems, which were limitations of the first law. This law introduces the concept of entropy and states that all non-equilibrium states of a system shift to an equilibrium state, eventually, in a natural way.
Entropy
Entropy (S) is a state function and the change in the entropy of both the system and surroundings gives us important conclusions regarding a system or reaction.

In irreversible or spontaneous processes:  i.e., the entropy is positive.
i.e., the entropy is positive.
Whereas in a reversible process,  . And when,
. And when,  we consider such a universe or system to be non-spontaneous.
we consider such a universe or system to be non-spontaneous.
The third law is also known as the zeroth law. It states that the entropy of a system is zero as the system approaches absolute zero temperature (0 K).
Also Read: What Is The ‘Big Freeze’ Hypothesis?
Gibbs Free Energy
From the second law of thermodynamics, we can deduce and understand the spontaneity and feasibility of a system/process. However, it becomes difficult to obtain the entropy of the surroundings. Thus, considering entropy as the sole criterion for the spontaneity of a process is not very convenient. In 1873, the paper published by Gibbs outlined an alternative, simpler criterion. This was done by introducing another thermodynamic function called free energy (G). The change in the free energy of the system could be the criterion to decide the spontaneity of the process and the free energy of the surroundings need not be taken into consideration.
When  , then the system is a spontaneous or product-favored process, as well as an irreversible process. On the other hand, if
, then the system is a spontaneous or product-favored process, as well as an irreversible process. On the other hand, if  , then the system is in an equilibrium state and is a reversible process. In cases where
, then the system is in an equilibrium state and is a reversible process. In cases where  , then such a system is a non-spontaneous process.
, then such a system is a non-spontaneous process.
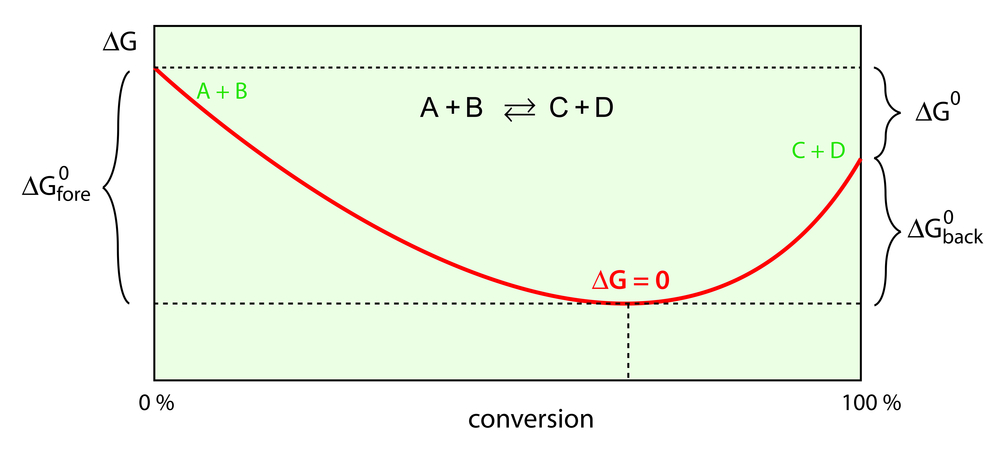
The Relation Between Gibbs Free Energy And Work Done
Theoretically, spontaneous reactions produce useful work. Their energy directly aids the occurrence of a reaction. When maximum useful work is obtained, such a reaction will not produce any entropy.
Thus: 
However, in reality, this useful work is always less than the maximum work, so there will be some entropy created.
When a system reaches the equilibrium state, it loses the capacity to perform external work. Since free energy of the system is the difference between the initial state energy and the equilibrium state energy, it is the energy available to do work. The equilibrium state energy is referred to as ‘non-available energy’, and is mathematically expressed as the product of temperature and entropy.
Mathematical Form For Gibbs Free Energy
 , at Constant Temperature and Pressure.
, at Constant Temperature and Pressure.
And as the reaction proceeds:

When a system acquires the state of maximum entropy (  ) and a minimum Gibbs free energy (
) and a minimum Gibbs free energy (  ), then the system has reached an equilibrium state. Thus, we can say that changes in heat and entropy are the two factors affecting Gibbs free energy, and in turn, the spontaneity of any physical or chemical change.
), then the system has reached an equilibrium state. Thus, we can say that changes in heat and entropy are the two factors affecting Gibbs free energy, and in turn, the spontaneity of any physical or chemical change.
Categories Of Chemical Reactions Based On Gibbs Free Energy
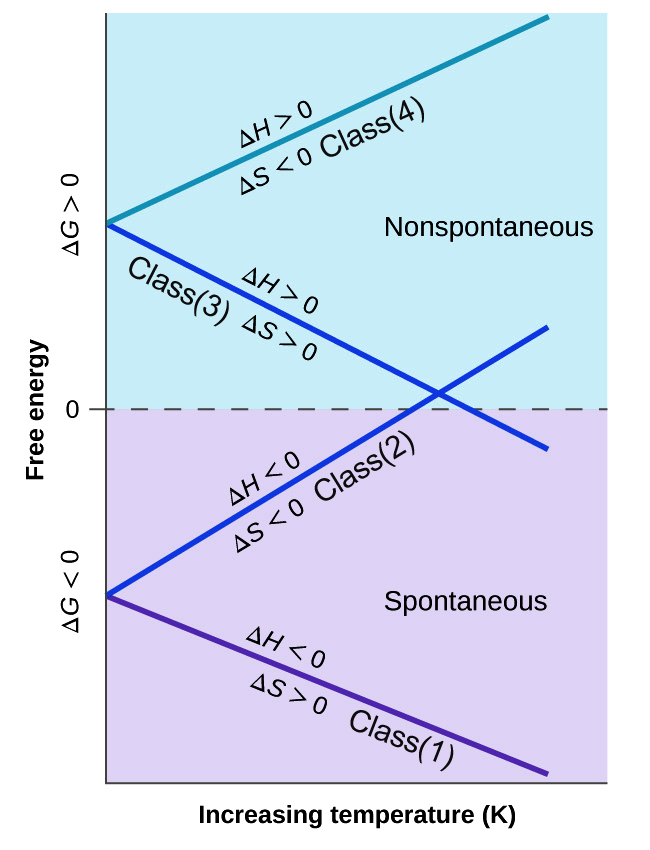
By knowing the variation of the Gibbs Free Energy, we can predict the spontaneity of a process. Different processes have different Gibbs Free Energy and based on their variation with temperature, we categorize them in four classes of reactions.
Class 1 reactions are spontaneous or product-favored at all temperatures. In Class 2 and 3 types of reactions, the spontaneity of the forward reaction depends on temperature. In Class 2, the reaction becomes product-favored at a lower temperature, while in Class 3, the product-favored reactions are at higher temperatures. Class 4 type reactions favor the reactants and are non-spontaneous for all temperatures.
Thus, the Gibbs free energy equation helps to estimate the temperature at which any process reaches equilibrium.
How well do you understand the article above!

