Table of Contents (click to expand)
Plastics are valuable, but to protect our much more valuable environment, we need an integrated approach, by combining alternative materials and advanced waste management techniques.
There’s a good chance that you’re reading this article on a mobile phone, about 40% of which is made of plastic.
Ranging from the ceiling fan above you to the corrective glasses you wear, virtually everything is made of plastic. Plastics have become a ubiquitous part of our everyday life, and a critical part of the world economy.

Why Is Plastic Popular?
It would not be wrong to say that the foundation of the modern world has been laid with bricks of plastic. They have led to a major disruption in the world of materials. You may wonder, what makes one material better than the other? We can find certain properties by which we can rank them.
- Their cost is low.
- In many cases, they are reusable.
- The manipulation of their properties is easy.
- They are relatively less toxic than other materials.
- Last but not least: the lack of any other material with all the above qualities, meaning there is no real competition.
Furthermore, plastics became widely used because of their durability, which simply means they are resistant to chemical and physical degradation. As we will see next, plastics are also directly related to the coal and petroleum industry. The cheap prices and popularity of these industries give plastics an extra edge.
Also Read: Are All Plastics Equally Harmful To The Environment?
How Did They Get Here?
Plastics, in general, refer to an array of polymers. Polymers have an organic backbone composed of carbon and hydrogen. These chains may additionally have side chains attached to them that can be interconnected to each other. Along with carbon and hydrogen, there are atoms of oxygen, nitrogen, sulfur, phosphorus etc. present in the chains.
Plastics are obtained from natural gas, coal and petroleum (this also makes them non-renewable). The process of manufacturing them involves several steps, such as refining, molding, curing, etc. and requires specific catalysts that increase the rate of the reactions. The key step in the whole process is polymerization. In this process, the monomers combine and join to form the chain characteristic of the plastic. If the monomers, polymers and all their chemistry is getting over your head, then we have an article ready to help explain the basics.
A Background Of Things
Even though plastics have many problems associated with them, they are still quite valuable. As a reference, in 2019, the global plastics market size was valued at USD 568.7 billion. Unfortunately, its large popularity has been accompanied by an even larger concern over pollution. However, while infamous, plastics are far from the only source of pollution. As a starter, the paper industry is linked with deforestation and water over-utilization, the coal industry is linked to air pollution, the fertilizer industry with water contamination and so on. In our world, the more important (and logical) question is “which thing is less polluting?”, rather than “which is polluting and which is not?”.

Also Read: Why Can’t We Burn Garbage In A Closed Environment That Doesn’t Let Fumes Escape?
Why It’s So Hard To Recycle Plastic?
As with everything else, nothing is ideal. At first, plastics seemed to have all the properties of an ideal material and thus became very popular. However, the more indispensable they became, the harder it turned out to dispense them! Among all the hurdles, a major problem is that plastics are cheap. Yes, you read that right (it’s a problem). They are inexpensive to manufacture, and recycled plastic is costlier than its virgin counterpart. Thus, it’s no wonder that companies don’t spend money segregating and recycling them because it’s more economical to manufacture them fresh.
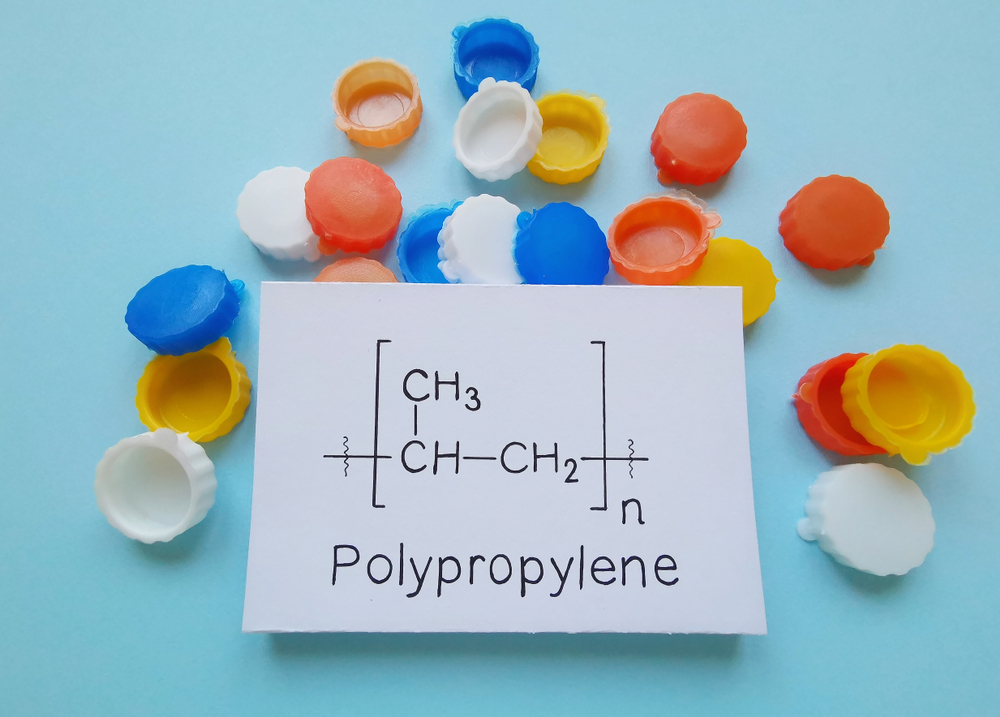
Continuing on this path is also related to the issue of variability, which is due to the easy manipulation of their properties. A huge variety of plastics like LDP, HDP (based on density), polystyrene, polypropylene etc. exist. Similar-looking plastics may be chemically different, which leads us to a large problem when it comes to segregation. Also, segregating manually makes the process more expensive. As a solution to the problem, a marking scheme has been implemented that makes identification simpler by using symbols.
And thus we come to the next gravest problem. Plastics are infamously hard to decompose. A plastic cup may remain for as long as 50 years in the soil without degrading! This is the dark side of plastic and the reason it is so closely tied to pollution. The constituent elements of plastics are interconnected by very strong bonds, which make them chemically inert. Strong bonds are energetically stable and require more energy to break. Therefore, decomposing microbes can’t use it as food, since there is no energy for them to consume in the first place. We have a more elaborate explanation of plastic decomposition processes here.

Also Read: How Do We Know Plastic Will Take So Long To Decompose?
Just A Solution
None of the substitute materials developed thus far offer a complete replacement for plastic (if one did, will it form a new plastic-type problem?). Until new materials and techniques come to the rescue, we can do our bit by substituting plastics with other materials when they are not necessary and segregating waste as much as possible.
Plastics take a long time to degrade, which is why there are millions of tons of plastic polluting the great oceans of the world, but there is also another side to it. What if those wastes weren’t thrown into the oceans in the first place? That question is so complex that it deserves an article of its own. For example, in 2010, about 8 million tons of plastic waste was dumped in the oceans around the world.
While alternative materials may have certain limitations of their own, the best approach we have is to stop disposing of plastic wastes in rivers, ponds, land, etc. (any place other than your recycling bin). Finally, spreading awareness will help solve the problem, which is what we strive for here at ScienceABC!
How well do you understand the article above!

